Researchers on the Division of Power’s Pacific Northwest Nationwide Laboratory have introduced a discovery. Regardless of forming in a wonderfully hexagonal lattice, ice displays stunning flexibility and malleability, which accounts for the frequent entrapment of fuel bubbles inside ice. These findings are derived from the inaugural molecular-resolution observations of nanoscale ice samples frozen from liquid water, which have been printed within the journal Nature Communications.
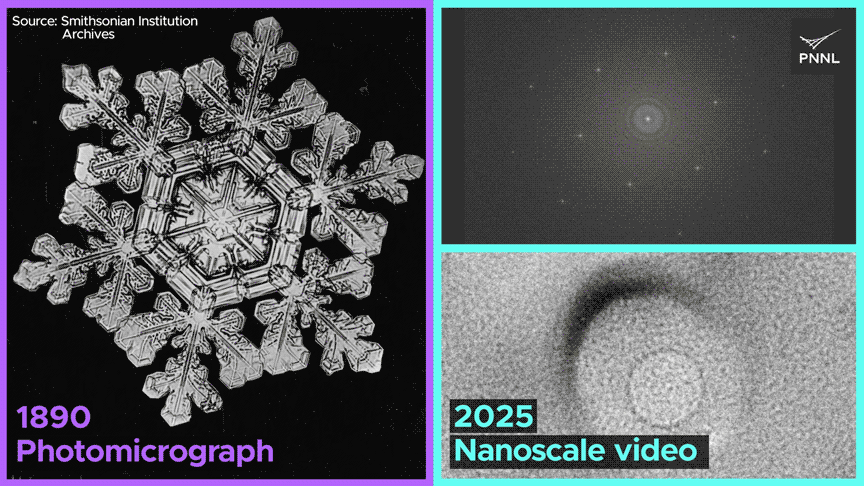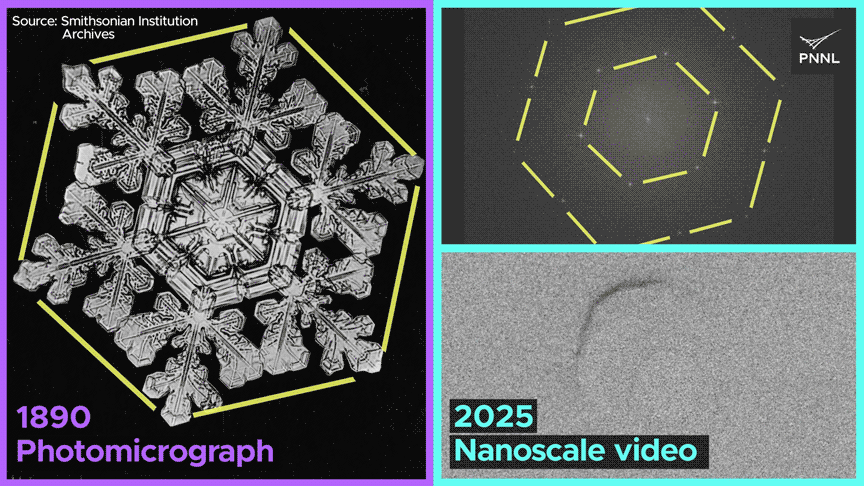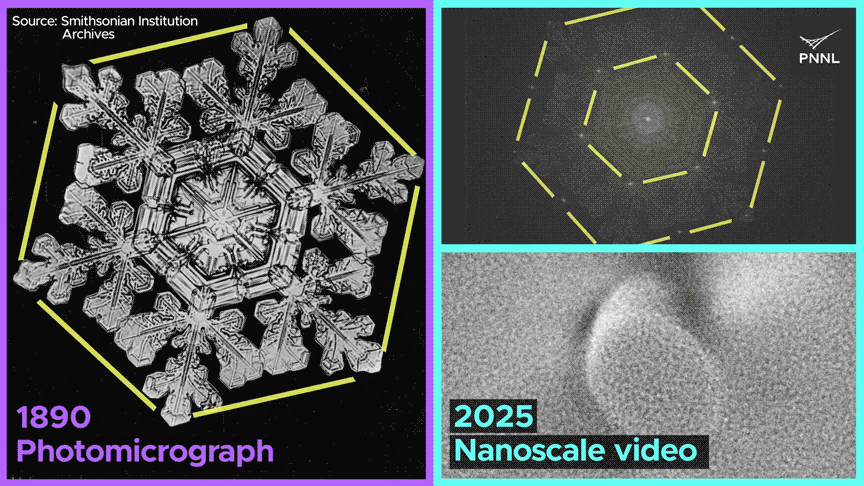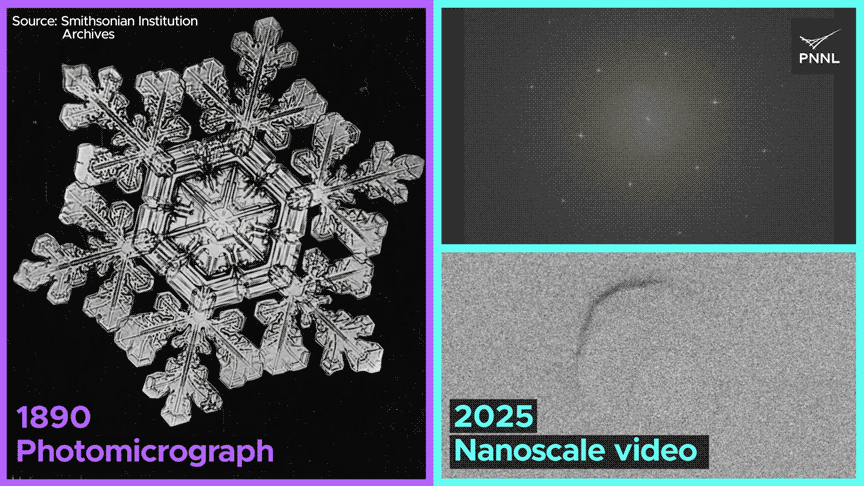
We noticed dissolved fuel not solely generate cavities in ice crystals, but in addition migrate, merge with different fuel bubbles and dissolve—conduct that’s solely potential because of the uncommon nature of bonding in ice. This work opens up a completely new alternative to discover ice crystallization and melting conduct at scales unimaginable just a few years in the past.
James De Yoreo, Research Principal Investigator and Battelle Fellow, Pacific Northwest Nationwide Laboratory (PNNL)
The research could have vital penalties for the preservation of cryogenically frozen organic tissue samples, predicting ice dynamics for the security of aviation and automobiles, and comprehending glacier motion, amongst varied different analysis domains.
There have been a variety of mysteries about ice. We wish to perceive how ice tolerates structural imperfections within the crystal and the way trapped bubbles have an effect on the mechanical properties of the crystal. Now we now have a technique to perceive that.
Jingshan Du, Research Lead Writer and Supplies Scientist, Pacific Northwest Nationwide Laboratory
What’s New With Ice
Nobody has succeeded in instantly observing water molecules transitioning from liquid to ice. It’s because the strategies employed by scientists to visualise particular person atoms require excessive situations, similar to the usage of high-energy radiation and the elimination of all air by means of vacuum sealing.
Though researchers have produced some pictures of ice on the molecular stage, these pictures don’t signify the everyday freeze-thaw cycles skilled on Earth. As a substitute, they’re created by means of a means of flash freezing that happens instantly from vapor to strong.
The analysis workforce positioned liquid water between skinny carbon membranes, which was the important aspect facilitating this imaging development. Subsequently, they devised a novel approach referred to as cryogenic liquid-cell transmission electron microscopy to watch the freezing course of.
“The membranes defend the ice crystals from excessive vacuum and radiation, permitting us to accumulate pictures with atomic-level data,” mentioned Du.
The workforce noticed the formation of fuel bubbles, their motion by means of the lattice, their merging with different bubbles, and their subsequent dissolution.
The research indicated that when liquid water transitions into strong ice, the defects inside its crystal construction or the presence of trapped fuel bubbles don’t induce vital pressure on the ice crystal, which might result in fracturing. It adjusts to the existence of those defects with exceptional ease compared to different solids, similar to metals or minerals.
The traits of water’s chemical bonds render it exceptionally versatile and malleable, even in its strong ice kind. This latest commentary, together with the important indisputable fact that ice is much less dense than liquid water, constitutes properties important for sustaining life on Earth, significantly in marine environments.
The researchers carried out direct observations of the geometries and forces that affect ice crystal formation throughout all scales, together with the event of snowflakes. Though snow originates from water vapor reasonably than liquid water, the identical basic forces are in operation.
The scientists at PNNL labored at the side of researchers from Argonne Nationwide Laboratory and the College of Illinois-Chicago, who had employed machine studying to create a extremely exact molecular dynamics mannequin for ice. The comparisons made between experimental outcomes and predictions from theoretical fashions affirmed that ice is distinct amongst solids in its potential to tolerate defects whereas sustaining the integrity of its crystal construction.
Why Trapped Air Bubbles in Ice Matter
Whereas the PNNL workforce investigates ice dynamics on the nanoscale, different researchers discover that air bubbles inside glaciers considerably affect their conduct. Lately, scientists demonstrated that glaciers soften over twice as quick once they include bubbles, in distinction to ice that is freed from bubbles. Moreover, different scientists are searching for to stop ice formation in delicate tissue samples or on plane throughout flight.
The forthcoming phases of this research will contain analyzing melting processes and dealing with extra advanced samples, similar to water containing dissolved substances.
Alongside Du and DeYoreo, PNNL researcher Ajay S. Karakoti; scientists Suvo Banik, Henry Chan, and Subramanian Okay. R. S. Sankaranarayanan from Argonne Nationwide Laboratory; Birk Fritsch and Andreas Hutzler from the Helmholtz Institute Erlangen-Nürnberg for Renewable Power; and Ying Xia from the College of Washington additionally performed a task within the analysis.
The research obtained funding from the DOE Workplace of Science, Fundamental Power Sciences, Division of Supplies Science and Engineering. The molecular dynamics simulations have been funded by the Knowledge, Synthetic Intelligence, and Machine Studying at Scientific Consumer Services program, which is a part of the Digital Twin Venture at Argonne Nationwide Laboratory.
A section of the research was carried out on the Environmental Molecular Sciences Laboratory, a scientific consumer facility at PNNL, in addition to on the Molecular Foundry and the Nationwide Power Analysis Scientific Computing Middle, each of that are DOE-supported consumer services positioned at Lawrence Berkeley Nationwide Laboratory.
Why ice cubes lure air bubbles
Why do ice cubes so typically lure air bubbles? Now we all know, because of researchers at Pacific Northwest Nationwide Laboratory, who captured the first-ever nanoscale pictures of ice crystals shaped from liquid water. Video Credit score: Animation by Sara Levine | Pacific Northwest Nationwide Laboratory.
Journal Reference:
Chan, H., et al. (2025) Molecular-resolution imaging of ice crystallized from liquid water by cryogenic liquid-cell TEM. Nature Communications. doi.org/10.1038/s41467-025-62451-0

