Synthesis and characterization of block copolymers and NPs
The artificial routes had been minutely depicted in Fig. S1. Initially, using a classical one-pot methodology [36], the diblock three-component copolymer with tunable stability, poly(cyclobutane−1,2,3,4-tetracarboxylic dianhydride-co-hydroxyethyl disulfide)-polyethylene glycol (mPEG5K–p(CBDA-co-BiBi)-mPEG5K, abbreviated as ssP-COOH), was synthesized, utilizing 2-hydroxyethyl disulfide (BiBi) monomer and cyclobutane−1,2,3,4-tetracarboxylic dianhydride (CBDA) molecular synthesis as useful skeletons, in addition to the introduction of mPEG5k-NH2 chain as hydrophilic fragment (Fig. S1). Primarily based on the nucleophilic reactions, thus, the as-prepared ssP-COOH copolymer displayed quite a few disulfide bonds (-S-S-) and pendent pairwise carboxylic acid (-COOH) teams, offering a basis for creating numerous copolymers. Subsequently, an normal EDC/HOBT-catalyzed amidation response was carried out to couple the aspect carboxylic acid group with ethylenediamine-monoboc (EDA-Boc), after which the modified copolymer was named as ssP-EDA-Boc copolymer. The following work was a easy in-situ Boc-deprotection response utilizing trifluoroacetic acid (TFA) resolution to kind an amino-modified polymer (ssP-EDA copolymer). The chemical buildings of those block copolymers had been characterised via 1H nuclear magnetic resonance (1H NMR) spectroscopy (Fig. S2) and fourier remodel infrared (FTIR) spectroscopy (Fig. S3) and UV-vis spectra (Fig. S4), confirming their profitable synthesis.
Moreover, it’s well-known that, in aqueous resolution, amphiphilic block copolymers can spontaneously drive the formation of numerous nanoparticles (similar to spheres, cylinders and lamellaes) via self-assembly method [36, 37]. Due to this fact, the distinct NPs, together with ssP-COOH, ssP-EDA-Boc, and ssP-EDA, had been fabricated in delicate resolution. The diameter of those NPs decreased progressively from 197.6 nm to 146.1 nm after which to 108.1 nm, as analyzed by DLS, as a result of gradual modification of copolymer (Fig. S5a, S5c), primarily in all probability as a result of elevated hydrophobic capability and electrostatic adsorption impact. Furthermore, variations in zeta potential additional proved the success of grooming (Fig. S5b).
To reinforce the sturdy energetic metallic (e.g., Fe3+) carrying capability of block copolymers, we integrated a polyphenol-like construction with acid-responsive Schiff-base bonding, using the three,4,5-trihydroxybenzaldehyde (tHB) monomer. A brand new peak appeared round 7.9–6.7 ppm within the 1H NMR spectrum was attributed to the benzene ring construction, indicating the profitable preparation of tHB-decorated block copolymer (ssP-tHB) (Fig. S2). Moreover, the presence of v (C = N) azomethine stretching vibrations was confirmed by the detection of absorption peaks at 1720 cm − 1 for tHB, which signifies the institution of Schiff base linkage [19].
Subsequent, the FDA-approved doxorubicin (DOX), as a hydrophobic broad spectrum anti-cancer drug, usually lacks bio-targeting, resulting in mobile resistance and limiting its effectiveness in most cancers therapy [19, 38]. To that finish, utilizing nano-precipitation, the ssP-tHB block copolymer created sq. nanoparticles that encapsulated DOX via hydrophobic interactions. These had been then enhanced with polyphenol-iron coordination to kind ssP-tHB@Fe/DOX (Fig. 1a), which boasts sturdy stability, minimal drug leakage, and excessive iron ion-carrying capability.
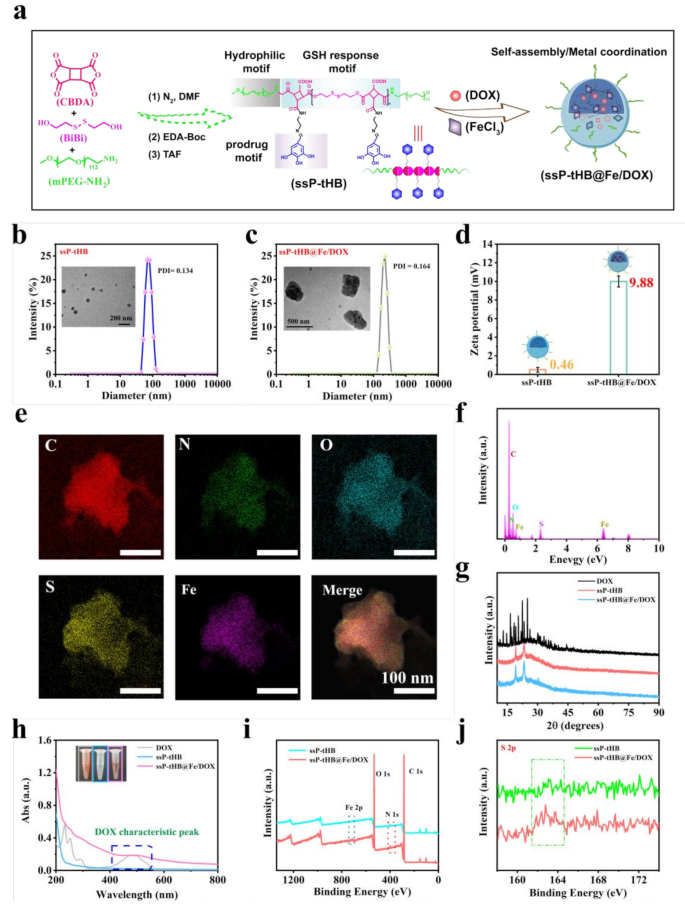
Preparation and characterization of ssP-tHB@Fe/DOX. (a) Synthesis routes of various nanoparticles. (b, c) Hydrodynamic sizes and TEM photos of ssP-tHB (b), and ssP-tHB@Fe/DOX (c). (d) Zeta potential modifications of nanoparticles (n = 5). (e) EDS elemental mapping photos of ssP-tHB@Fe/DOX. (f) EDS components of ssP-tHB@Fe/DOX. (g) Powder XRD patterns of free DOX, ssP-tHB, and ssP-tHB@Fe/DOX. (h) UV-vis absorption spectra of free DOX, ssP-tHB, and ssP-tHB@Fe/DOX. Inset: The corresponding pictures of samples. (i) XPS spectra of ssP-tHB and ssP-tHB@Fe/DOX. (j) Excessive-resolution S 2p XPS spectra of ssP-tHB and ssP-tHB@Fe/DOX
After that, the composition and construction of ssP-tHB and Fe/DOX-coloaded ssP-tHB had been minutely characterised. By DLS testing, in aqueous resolution, the self-assembled ssP-tHB block copolymer prominently fashioned spherical nanoparticles with a hydrodynamic particle measurement of 147 nm and a polydispersity index (PDI) of 0.134 (Fig. 1b). Fe/DOX-loaded NPs have a bigger hydrodynamic diameter of roughly 220 nm and a PDI of 0.164 in comparison with clean NPs, demonstrating profitable payload incorporation (Fig. 1c).
In TEM photos, ssP-tHB@Fe/DOX in tough tablet-like morphology with about 350 nm in size and 200 nm in width was confirmed, whereas clean NPs had been uniformly spherical, measuring 50–60 nm (Fig. 1b), indicting that DOX loading and iron ions coordination can affect the morphology of the nanoassemblies. Moreover, the self-assembled ssP-tHB resolution shrunk in its dry state as a result of dehydration impact [19, 20]. Nevertheless, this related phenomenon didn’t happen with ssP-tHB@Fe/DOX, primarily as a result of iron-phenol crosslinking stabilized the nanoparticle construction.
The zeta potential of ssP-tHB decreased from 13.42 mV (ssP-EDA) to 0.46 mV, attributed to the detrimental costs of tHB pendant teams [39]. In the meantime, a positively charged ssP-tHB@Fe/DOX (9.88 mV) was noticed in comparison with clean NPs (ssP-tHB) (Fig. 1d), primarily as a result of profitable incorporation of the positively charged DOX drug and iron ions. As confirmed in UV-vis absorption spectra (Fig. 1h), ssP-tHB illustrated no apparent attribute absorption peak. Conversely, the absorption peak at 410–550 nm, attribute of ssP-tHB@Fe/DOX or DOX, signifies the profitable incorporation of DOX into the nanoplatform.
To research the loading of DOX and iron ions, we subsequently performed a collection of experiments. Within the research, via resolution self-assembly, the anticancer drug DOX was encapsulated, adopted by the introduction of trivalent iron ions for metallic coordination to additional stop leakage of DOX and improve the soundness of NPs. Due to this fact, the ready nanoparticles (ssP-tHB@DOX) had a mean particle measurement of roughly 165 nm, with a PDI of 0.121 and a zeta potential of 4.56 mV, by way of DLS measurement (Fig. S6a), indicating that the hydrophobic chemotherapeutic drug DOX was primarily encapsulated inside the hydrophobic area of block copolymers via self-assembly methodology. Moreover, the UV-vis spectra of various samples had been analyzed. As proven in Fig. S6b, after mixing free DOX with Fe3 + resolution, the attribute peak of DOX at about 480 nm didn’t fluctuate, indicating that the chelation between iron ions and DOX didn’t intrude with the attribute absorption peak of DOX. Subsequently, when ssP-tHB@Fe/DOX was added in DMSO resolution, it was clearly noticed via digital pictures that the pink coloration of the DMSO resolution turned deeper in comparison with in aqueous resolution, and the attribute peaks of DOX additionally enhanced by UV-vis spectra (Fig. S6c), indicating that the DMSO resolution successfully disrupted the polymer-based nanoparticles, selling the discharge of DOX from the hydrophobic cavities of the copolymer. Moreover, the DMSO-treated ssP-tHB@Fe/DOX was divided into two segments, with sizes starting from 40 to 68 nm and 531–955 nm (Fig. S6d), additional demonstrating that DMSO resolution may successfully disrupt NPs stability. Because of this, the weakening of DOX’s attribute peaks after drug loading might be attributed to: (a) efficient incorporation of the hydrophobic chemotherapeutic anticancer drug DOX into the polymer’s hydrophobic layer throughout the resolution self-assembly course of; (b) interference with DOX’s attribute absorption peak as a consequence of π-π stacking interactions involving its massive benzene rings [20, 39]. Concurrently, Fe3 + primarily utilized the coordination between phenol group and iron ions to successfully carry iron ions on the copolymer floor.
Based on the DOX concentration-dependent absorption normal curve, the content material of DOX in ssP-tHB@Fe/DOX estimated to be 21.2% (Fig. S7). Furthermore, the mass share of Fe in ssP-tHB@Fe/DOX about was 17.3% via ICP expertise. The Fig. 1e confirmed the distribution of components for ssP-tHB@Fe/DOX, and associated components similar to C, N, O, S and Fe had been evenly dispersed on its the floor, indicating the profitable preparation of nanoparticles. As well as, the energy-dispersive X-ray (EDC) spectrum additional corroborated this discovering (Fig. 1f). A consequent X-ray photoelectron spectroscopy (XPS) characterization additional confirmed the development of the ssP-tHB@Fe/DOX (Fig. 1i). In the meantime, the standard peak about at 163.8 eV listed as S-S or S-C bonds [40], was noticed in S subtle XPS spectra of ssP-tHB and ssP-tHB@Fe/DOX, successfully confirming the presence of nanoparticles aware of GSH (Fig. 1j). Following this, we carried out an XRD evaluation on the crystal buildings of ssP-tHB, ssP-tHB@Fe/DOX, and free DOX powder. The outcomes indicated that each the answer self-assembled nanoparticles and the DOX drug exhibited an amorphous construction [39]. Throughout self-assembly, the attribute diffraction peaks at 10º−40º of DOX vanished in comparison with ssP-tHB or ssP-tHB@Fe/DOX (Fig. 1g), possible as a consequence of π-π stacking and hydrophobic interactions between them [39].
GSH-responsive degradation and catalytic efficiency
The favorable colloidal stability and applicable measurement of nanoparticles are essential elements for the buildup in tumor areas enhanced by the permeability and retention (EPR) impact [19, 41]. We additional investigated the soundness of ssP-tHB@Fe/DOX in numerous resolution (PBS, 10% FBS medium, and dH2O) (Fig. 2a). There have been no important modifications in particle distribution throughout totally different media. Moreover, the hydrodynamic diameters of ssP-tHB@Fe/DOX remained fixed over the seven-day monitoring experiment (Fig. 2b). This establishes a basis for utilizing ssP-tHB@Fe/DOX in tumor remedy via caudal intravenous injection.
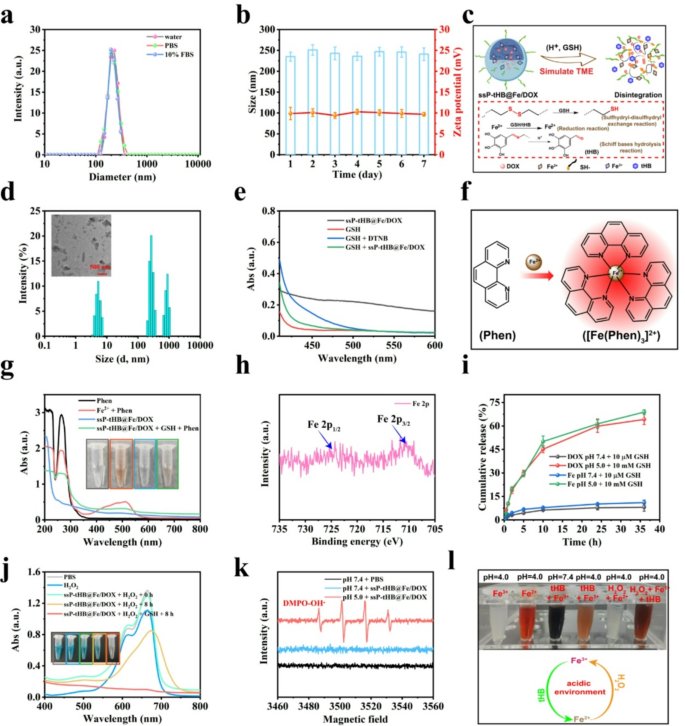
GSH responsive efficiency and ·OH technology assessments. (a) Hydrodynamic measurement of ssP-tHB@Fe/DOX in numerous media (water, PBS, and 10% FBS) (n = 5). (b) Modifications of measurement and zeta potential of ssP-tHB@Fe/DOX in PBS for 7 days (n = 5). (c) The diagram of H+/GSH-emitted degradation and launch of ssP-tHB@Fe/DOX. (d) Modifications in hydrodynamic measurement of ssP-tHB@Fe/DOX in pH 5.0 + 10 mM GSH at 12 h. Inset: The corresponding TEM picture of samples. (e) UV-vis spectra for GSH stage detection utilizing DTNB as a probe within the presence of ssP-tHB@Fe/DOX. (f) Schematic illustration for Fe2+ detection utilizing 1,10-Phenanthroline (Phen) probe. (g) UV-vis spectra for Fe2+ detection utilizing 1,10-phenanthroline as a probe in numerous mixtures. Inset: The corresponding images of samples (from left to proper). (h) Excessive-resolution XPS spectrum of Fe 2p in ssP-tHB@Fe/DOX. (i) The discharge profile of DOX and Fe ions from ssP-tHB@Fe/DOX underneath totally different stimulation circumstances (n = 5). (j) EPR spectra of DMPO-OH in aqueous options in numerous circumstances. (ok) UV-vis absorption spectra of concerning ssP-tHB@Fe/DOX-mediated MB degradation underneath totally different circumstances. (l) Digital pictures displaying interactions between iron ions and tHB molecules underneath totally different circumstances
Present analysis signifies that tumor areas show important variations from regular tissues, similar to elevated ranges of GSH, an acidic surroundings, and varied enzymes that promote tumor progress [16, 19]. Due to this fact, these tumor-specific nurturing microenvironments will improve the effectiveness of clever nanotechnology supply methods, notably tumor-responsive drug launch. Primarily based on this, the nanoparticles containing S-S bonds and Fe3 + ions had been assessed underneath GSH circumstances (Fig. 2c). As depicted in Fig. 2d, ssP-tHB@Fe/DOX undergone extreme disintegration at pH 5.0 with 10 mM GSH over 12 h, as noticed via TEM imaging. DLS evaluation revealed three distinct particle measurement alerts for acid/GSH-treated ssP-tHB@Fe/DOX: small fragments (5–10 nm), bigger particles (300–400 nm), and micron-sized particles. This implies that acid and GSH circumstances may cause the degradation of ssP-tHB@Fe/DOX. We then explored the capability of GSH consumption of ssP-tHB@Fe/DOX or ssP-tHB via the 5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB) chromogenic methodology (Fig. 2e, S8). Nanoparticles containing plentiful S-S bonds confirmed a sturdy GSH-depleting functionality, relying primarily on the presence of nanoparticle-rich disulfide bonds and high-valence iron ions. So, these findings point out that ssP-tHB@Fe/DOX has GSH-triggered ferroptosis-inducing potential for tumor therapy.
Consequently, the essential function of iron within the induction of ferroptosis was examined. Due to this fact, the 1,10-phenanthrolline (Phen) probe was chosen for detecting bivalent iron ions in ssP-tHB@Fe/DOX utilizing UV-vis spectroscopy (Fig. 2f). The pink flocculation was clearly confirmed when GSH handled ssP-tHB@Fe/DOX, indicating the manufacturing of Fe2 + by GSH discount (Fig. 2g). The high-resolution XPS spectrum of Fe 2p revealed that Fe in ssP-tHB@Fe/DOX exists as each Fe(II) and Fe(III). Particularly, Fe 2p3/2 and Fe 2p1/2 peaks had been noticed at 711.4 eV and 727.5 eV [42], respectively (Fig. 2h), indicating tHB-induced technology of Fe2 + from ssP-tHB@Fe/DOX. Thus, we additionally investigated the chemical transformations of small molecule tHB and iron ions in varied oxidation states throughout totally different media (Fig. 2l). The conversion of Fe3+/Fe2 + was examined by a 1,10-phenanthroline probe (Fig. 2f). Underneath acidic circumstances (pH 5.5), tHB and trivalent iron ions exhibited a pale brown coloration, in contrast to the Fe(II) resolution. At a pH of 5.5, the answer of Fe(II) exhibited a pink coloration change upon the introduction of tHB, indicating the discount of Fe(II) by tHB. Apparently, the presence of H2O2 resulted in each Fe (II) and tHB small molecules displaying related pink colours. Thus, we are able to anticipate that the tHB-mediated Fe3+-to-Fe2 + conversion following ssP-tHB@Fe/DOX internalization by tumor cells will guarantee steady Fe2 + manufacturing, facilitating extremely environment friendly Fenton reactions [43].
Subsequent, given the excessive GSH expression in tumors that mediates the response to ssP-tHB@Fe/DOX, the drug launch curves of the loaded DOX and iron ions had been investigated (Fig. 2c). As proven in Fig. 2i, in pH 5.0 and 10 mM GSH (simulating tumor microenvironments), DOX from ssP-tHB@Fe/DOX displayed a steady launch motion, attaining a cumulative launch charge of 64.2% in 36 h, whereas solely 11.3% of DOX was launched throughout incubation in pH 7.4 and 10 µM GSH (simulating regular circumstances) over the identical time interval. As well as, for iron ions in ssP-tHB@Fe/DOX, the analogous launch profiles had been collected by ICP testing, and roughly 68.9% of iron ions had been launched underneath simulated tumor circumstances, whereas solely 11.1% was launched within the simulated regular circumstances (Fig. 2i). Some elements contribute to those behaviors: (a) ssP-tHB@Fe/DOX comprises -S-S- bonds that create the hydrophobic area resulting in disassembly and the discharge of DOX and iron ions; (b) Fe (III) is lowered to Fe (II), weakening its coordination with polyphenol teams and accelerating launch; (c) the Schiff base bonds connected to the tHB monomer detach within the presence of weak acid (pH 5.0), accelerating the disintegration of ssP-tHB@Fe/DOX by destroying the hydrophobic layer (Fig. 2c).
The discharge of iron ions from ssP-tHB@Fe/DOX induced the technology of extremely poisonous hydroxyl radicals (·OH) by way of the Fe-mediated Fenton response. Methylene blue (MB), used as a colorimetric substrate, was chosen for detection. As depicted in Fig. 2j, no apparent·OH formation was noticed in a pH 7.4 surroundings with 10 mM H2O2 after 8 h, indicating the non-toxic nature of ssP-tHB@Fe/DOX previous to reaching the tumor website. Nevertheless, underneath related tumor circumstances (pH 5.0 + 10 mM H2O2), ssP-tHB@Fe/DOX produced a substantial abundance of ·OH, ensuing within the oxidation of MB. Amusingly, wealthy ·OH was detected when 10 mM GSH was current (resembling the tumor intracellular surroundings), indicating that GSH-treated Fe (III) produced energetic Fe (II) to generate ·OH, additional demonstrating that ssP-tHB@Fe/DOX has tumor-activated therapeutic property. Electron spin resonance (ESR) spectroscopy, using 5.5-dimethylpyrroline N-oxide (DMPO), was employed to find out ·OH ranges (Fig. 2ok). We discovered that pH 7.4 surroundings was not liable for the manufacturing of ·OH, whereas iron-containing nanoparticles in pH 5.0 situation may generate considerable ·OH via the iron-based Fenton response in a manually simulated tumor microenvironments [44], indicating that its potential to induce ferroptosis in tumors.
Apoptosis and ferroptosis mechanism evaluation in vitro
At current, therapy mannequin of chemotherapy alone is susceptible to permit poor tumor efficacy and chemoresistance of tumor cells, thereby ensuing within the failure of tumor therapy or tumor development. Due to this fact, non-apoptotic dying property, particularly ferroptosis, was efficiently mixed to coordinate tumor remedy, on this research (Fig. 3h). It’s well-known that iron accumulation and subsequent lipid peroxidation (LPO) play essential roles in triggering and accelerating ferroptosis. Due to this fact, the molecular pathways and signaling mechanisms concerned in iron homeostasis and LPO play essential regulatory roles on this course of [26, 32, 40]. To this finish, we evaluated the iron uptake in Fe-containing nanoparticles (ssP-tHB@Fe/DOX) to not directly perceive the buildup of iron ranges in 4T1 cells and consider the power of Fe-induced ferroptosis. As introduced in Fig. 3b, the time-dependent uptake of iron ions in 4T1 cells confirmed that ssP-tHB@Fe/DOX might be internalized by cells and resided inside cells, laying the inspiration for inducing ferroptosis. Moreover, iron ions alone additionally confirmed a rise in time-dependence, probably as a result of presence of tumor cells with ferrophilic impact.
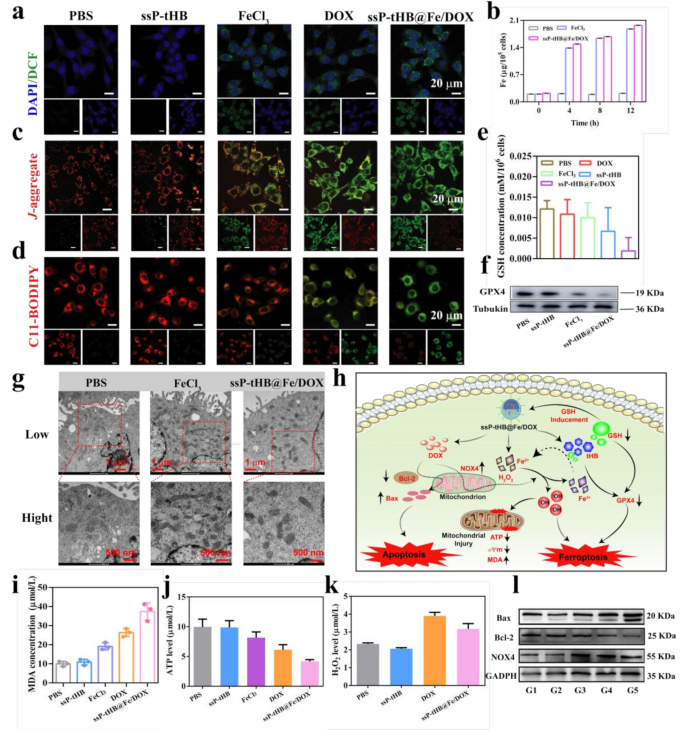
Antitumor mechanisms of ssP-tHB@Fe/DOX. (a) ROS ranges (indicated by DCFH-DA) in 4T1 cells after varied therapies utilizing CLSM. (b) Evaluation of intracellular Fe2+ content material in 4T1 cells at totally different time factors (0, 4, 8, and 12 h) after incubation with PBS, FeCl3, and ssP-tHB@Fe/DOX. (c) Confocal microscopic photos of 4T1 cells stained with JC-1 probes to guage intracellular mitochondrial membrane potential. (d) CLSM photos of C11-BODIPY-stained 4T1 cells after totally different therapies. Scale bar = 20 μm. (e) Evaluation of the GSH/GSSG ratio in cells. (f) Western blot evaluation of GPX4 expression. (g) Consultant bio-TEM photos of 4T1 cells. (h) The proposed schematic diagram of ssP-tHB@Fe/DOX-induced ferroptosis/apoptosis. (i, j, and ok) Intracellular MDA ranges (i), ATP content material (j), and H2O2 focus (ok) of 4T1 cells after totally different therapies. (l) Western blot evaluation of the expressions of Bax, Bcl-2, and NOX4. Be aware: PBS (G1), ssP-tHB (G2), FeCl3 (G3), DOX (G4), and ssP-tHB@Fe/DOX (G5)
Subsequently, therapies by totally different samples of 4T1 intracellular GSH ranges had been examined via GSH/GSSG assay equipment. Determine 3e indicated that GSH ranges considerably decreased in comparison with the PBS group following therapy with varied samples, together with DOX, FeCl3, ssP-tHB, and ssP-tHB@Fe/DOX. As anticipated, the consumption of endogenous GSH ranges was optimum throughout therapy with ssP-tHB@Fe/DOX in comparison with different teams. This phenomenon occurred as a consequence of two primary causes: (i) the involvement of discount reactions in teams containing Fe3+, and (ii) the consumption of nanoparticles via a sulfhydryl-dissulfhydryl alternate response with GSH.
Moreover, in accordance with earlier stories, the consumption of GSH disrupts the equilibrium of intracellular redox system, resulting in the emergence of cell oxidative stress and LPO [45]. We primarily monitored the technology of intracellular ROS using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) staining. As proven in Fig. 3a, the sturdy depth of inexperienced fluorescence from DCF was displayed after incubation of ssP-tHB@Fe/DOX. Moreover, different handled teams (FeCl3 and DOX) additionally confirmed low depth of inexperienced sign in comparison with management group or ssP-tHB, demonstrating that the technology of ROS. To research the manufacturing of •OH by way of iron-mediated Fenton response inside tumor cells, the fluorescence probe of HPF (indicating intracellular •OH ranges) was employed [46]. As proven in Fig. S9, a notable improve in HPF inexperienced fluorescence was noticed within the ssP-tHB@Fe/DOX group in comparison with PBS, ssP-tHB, FeCl3, and DOX teams. This evaluation additional confirmed that ssP-tHB@Fe/DOX-treated 4T1 cells induced the technology of considerable •OH via iron-mediated Fenton response, resulting in oxidative harm of cell membranes and triggering ferroptosis.
Owing to the sturdy oxidative capability of ROS in 4T1 cells, intracellular polyunsaturated fatty acids (PUFA) readily endure oxidation, forming lipid peroxides (LPO), which ends up in cell ferroptosis [45]. Due to this fact, we chosen C11-BODIPY581/591 probe to detect LPO using modifications within the excitation emission wavelength in cells, to evaluate its skill to induce ferroptosis. Determine 3d demonstrated that pink fluorescence decreased (discount state) and inexperienced fluorescence elevated (oxidized kind) following ssP-tHB@Fe/DOX therapy, in comparison with different therapy teams, indicating the strongest LPO exercise. Furthermore, DOX-treated 4T1 cells demonstrated the same stage of LPO exercise, aligning with the noticed manufacturing of ROS. As anticipated, by malondialdehyde (MDA) focus in 4T1 cells testing, related outcomes had been noticed (Fig. 3i). Because the Fe-mediated Fenton response relies on excessive ranges of H2O2, we investigated the manufacturing of H2O2 in 4T1 cells by varied samples. As already recognized, nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4), primarily derived from mitochondria, effectively generates H2O2 by catalyzing the switch of electrons from NADPH to oxygen [47, 48]. In early experiments, DOX not solely induces cell apoptosis but in addition triggers oxidative stress by growing the extent of H2O2. A big quantity of H2O2 was produced throughout DOX therapy, contributing to the ROS technology in tumor cells induced by DOX [49]. As well as, DOX-containing nanoparticles additionally produced considerable H2O2, in comparison with clean carriers (ssP-tHB) (Fig. 3ok). Due to this fact, within the tumor microenvironments, the circulatory amplification therapeutic methods had been fashioned, on the one hand, DOX induced chemotherapy, and on the opposite, DOX-assisted H2O2 manufacturing additionally aided within the amplification of ferroptosis.
The morphology of mitochondria in 4T1 cells handled with ssP-tHB@Fe/DOX was examined utilizing bio-TEM. In comparison with the PBS group, the ssP-tHB@Fe/DOX-treated mitochondria exhibited typical ferroptosis traits, together with smaller measurement, elevated membrane density, lowered or absent mitochondrial ridges, and disrupted outer mitochondrial membranes [50] (Fig. 3g). Determine 3c confirmed CLSM photos of 4T1 cells after therapies of various samples and stained with 5,5′,6,6′-tetrachloro−1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide (JC−1) to observe the intracellular mitochondrial membrane potential (MMP). The JC−1 confirmed pink fluorescence after therapy with PBS or ssP-tHB, indicating a state of aggregation inside mitochondria. Nevertheless, the JC−1 confirmed elevated inexperienced fluorescence after therapy with free FeCl3 or DOX alone, and probably the most intense inexperienced fluorescence when handled with ssP-tHB@Fe/DOX, suggesting the presence of monomers inside mitochondria in 4T1 cells and indicating the sturdy •OH technology functionality of ssP-tHB@Fe/DOX. This happens as a result of the •OH can hurt the mitochondria, leading to decreased MMP.
In depth research have recognized glutathione peroxidase 4 (GPX4) as a basic indicator of ferroptosis [23, 24]. Abnormalities in intracellular mitochondrial exercise, notably in power metabolism, had been noticed as a consequence of ssP-tHB@Fe/DOX-induced ferroptosis. The laboratory outcomes indicated a major lower in ATP ranges in 4T1 cells throughout ssP-tHB@Fe/DOX cultivation, in comparison with the management group. Different therapy teams (ssP-tHB, free FeCl3, and DOX alone) additionally confirmed the discount of ATP ranges (Fig. 3j). To raised perceive the mechanisms of ssP-tHB@Fe/DOX-induced cell dying, particularly apoptosis and ferroptosis, we investigated the associated pathways in pre-treated 4T1 cells. The outcomes of the western blot (WB) assays visually demonstrated that ssP-tHB@Fe/DOX inhibits GPX4 expression in vitro (Fig. 3f). Within the free FeCl3 therapy group, there was a decrease discount in GPX4 ranges in comparison with the traditional group, which mirrored the outcomes of GSH consumption as a consequence of their optimistic correlation [39]. Moreover, the lower in GXP4 expression in clean ssP-tHB additionally might be attributed to the consumption of GSH. Therefore, the expression of ranges of NADPH oxidase 4 (NOX4) in cells was investigated. As illustrated in Fig. 3l, S10c, DOX (G4) distinctly raised an enhancement in NOX4 stage compared to the management group. Moreover, Moreover, therapy with ssP-tHB (G2) and iron ions (G3) confirmed minimal change. Nevertheless, throughout the conversion of low-toxicity H2O2 into high-toxicity ·OH, ssP-tHB@Fe/DOX-treated mitochondria additionally skilled a sure diploma of mitochondrial dysfunction, similar to mitochondrial shrinkage and dissipation of membrane potential. Due to this fact, via WB evaluation (Fig. S10c), we intriguingly noticed that free DOX-induced NOX4 stage was barely increased than these induced by ssP-tHB@Fe/DOX. Subsequently, the affect of ssP-tHB@Fe/DOX on apoptotic pathways in 4T1 cells was additional investigated. As illustrated in Fig. 3l, S10a, and S10b, G5 considerably mediated the rise in pro-apoptotic protein Bax and the lower in anti-apoptotic protein Bcl−2. In different therapeutic teams, similar to G2, G3, and G4, there was a lower in anti-apoptotic Bcl−2 proteins and a rise in pro-apoptotic Bax proteins in comparison with the management group, indicating their skill to induce apoptosis to some extent.
Mobile internalization and cytotoxicity assays in vitro
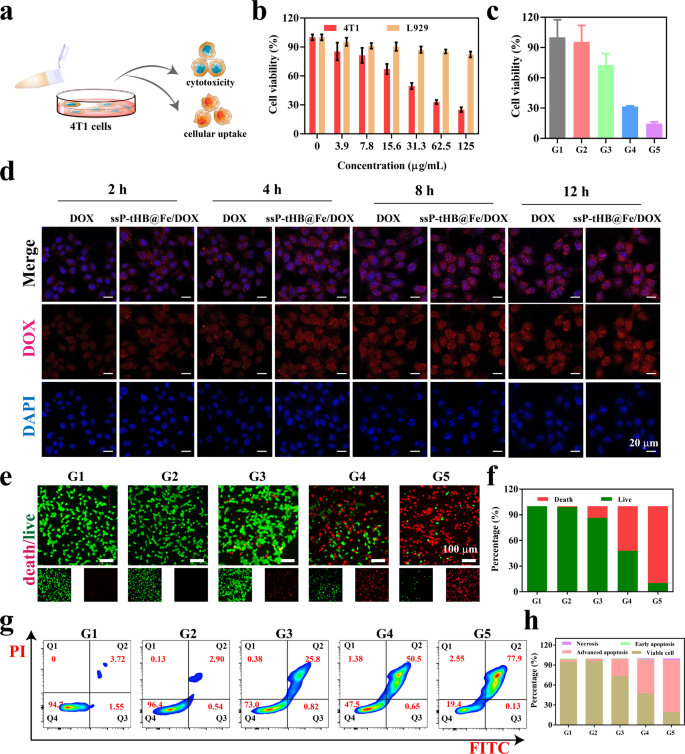
Subsequent, related cytotoxicity experiments in vitro had been evaluated via CCK−8 assays (Fig. 4a). Clean ssP-tHB and tHB-treated 4T1 cells confirmed important biocompatibility, even at a focus of 640 µg/mL, with solely a minor lower in cell viability (Fig. S11). Subsequently, the traditional L929 cells had been used to research the tumor-specific traits of ssP-tHB@Fe/DOX and free DOX (Fig. 4b, S12). After 24 h of incubation, the CCK−8 measurement outcomes confirmed that growing concentrations of ssP-tHB@Fe/DOX confirmed minimal cytotoxicity, demonstrating important biosafety for regular cells. Nevertheless, as a result of spectral anti-cancer impact of free DOX and its potent cytotoxicity, wholesome L929 cells exhibited concentration-dependent cytotoxic results (Fig. S12). Moreover, a dose-dependent cytotoxicity impact on 4T1 cells was exhibited within the presence of ssP-tHB@Fe/DOX, additional indicating its tumor-specific therapeutic potential (Fig. 4b). As proven in Fig. 4c, trivalent iron ions (G3) additionally exhibited a level of anti-tumor impact, with a cell survival charge of 72.7%, which can be attributed to iron-induced cell dying via the Fenton response. In DOX-treated experiments (G4) at equal doses, ssP-tHB@Fe/DOX (G5, 125 µg/mL) demonstrated considerably larger cytotoxicity in comparison with free DOX.
In vitro cytotoxicity associated evaluation. (a) The viability of 4T1 cells after totally different ssP-tHB concentrations therapy. (b) Cell viability of 4T1 cells and L919 cells handled with ssP-tHB@Fe/DOX for twenty-four h. (c) Relative viability of 4T1 cells in numerous samples. (d) Mobile uptake detected by CLSM. (e) CLSM photos of Calcein AM/PI-stained 4T1 cells after totally different therapies. (f) Quantification of dwell and useless cells after therapy. (g) 4T1 cell apoptosis assessed via annexin V-FITC and PI staining, analyzed by FCM. (h) Histogram displaying quantification of various apoptotic kinds. Be aware: PBS (G1), ssP-tHB (G2), FeCl3 (G3), DOX (G4), and ssP-tHB@Fe/DOX (G5)
Mobile uptake habits is taken into account a vital indicator for assessing the anti-tumor efficacy of “nano-vehicles” [51]. Due to this fact, as free DOX or ssP-tHB@Fe/DOX-cultured time elevated, the depth of pink fluorescence alerts turned stronger by CLSM photos (Fig. 4a and d). Concurrently, free of charge DOX, 4T1 cells confirmed a time-dependent improve in fluorescence. Importantly, because the tradition time will increase, pink fluorescence (DOX) overlapped with blue fluorescence (nucleus), indicating that DOX launched from ssP-tHB@Fe/DOX had moved from the cytoplasm to the nucleus for chemotherapy. The impact of phagocytosis of ssP-tHB@Fe/DOX was extra pronounced in comparison with free DOX, possible as a result of enhanced mobile uptake facilitated by nanoparticles.
Calcein-AM (inexperienced fluorescence for dwell cells) and propidium iodide (PI, pink fluorescence for useless cells) had been used collectively to distinguish between dwell and useless 4T1 most cancers cells. As proven in Fig. 4e and f, after processing with clean ssP-tHB (G2), practically all inexperienced fluorescence alerts had been noticed, indicating its non-toxic nature. Free FeCl3 (G3) and DOX (G4) had been discovered to kill cells to some extent, however their therapeutic impact was restricted in comparison with the ssP-tHB@Fe/DOX. The outcomes aligned with the conclusions of the CCK−8 assay.
Provided that the chemotherapy drug DOX successfully induces apoptosis in tumor cells [42, 47], we additionally examined the apoptosis of various samples on 4T1 cells utilizing circulate cytometry (Fig. 4g and h). In comparison with the management group (G1) or clean NPs (G2), the proportion of apoptotic cells considerably elevated within the FeCl3-treated group (G3). Moreover, free DOX (G4) confirmed sturdy cell apoptosis, with the apoptosis charge of fifty.5%. Of notice, the cell apoptosis charge rose to 77.9% after therapy with ssP-tHB@Fe/DOX (G5).
Hemolysis and acute toxicity take a look at in vivo
Impressed by the sensible efficiency of ssP-tHB@Fe/DOX in opposition to 4T1 cells in vitro, its anti-tumor efficacy was additional assessed in vivo. To research the biosafety of ssP-tHB@Fe/DOX, we carried out hemolysis assessments and acute toxicity measurements in vivo. The hemolysis charges had been low, at lower than 10%, in comparison with the management group. At a focus of 200 µg/mL, the ssP-tHB@Fe/DOX precipitated a hemolysis charge of solely 8.78%, indicating that the erythrocyte construction remained regular (Fig. S13). Because of this, ssP-tHB@Fe/DOX, with its tumor-specific remedy, demonstrated important blood compatibility and could be additional utilized in in vivo bio-related assessments. Subsequently, feminine Balb/c mice had been randomly divided into two teams (n = 6), after which injected intravenously with PBS (management group), ssP-tHB@Fe/DOX (DOX: 6 mg/kg, 100 µL), respectively. After 8 and 14 days of therapy, all mice had been euthanized, and their main organs (together with the guts, liver, spleen, lung, and kidney) had been eliminated for hematoxylin and eosin (H&E) staining (Fig. S14), and blood was additionally collected from their eyeballs for biochemical evaluation (Tables S1, S2) [52]. The H&E evaluation revealed no important organ harm or irritation within the ssP-tHB@Fe/DOX-treated mice. Moreover, ssP-tHB@Fe/DOX injection had no impact on liver and kidney features or blood parameters. In abstract, the GSH/pH dual-responsive ssP-tHB@Fe/DOX confirmed important potential for organic functions.
RNA-seq evaluation of ssP-tHB@Fe/DOX
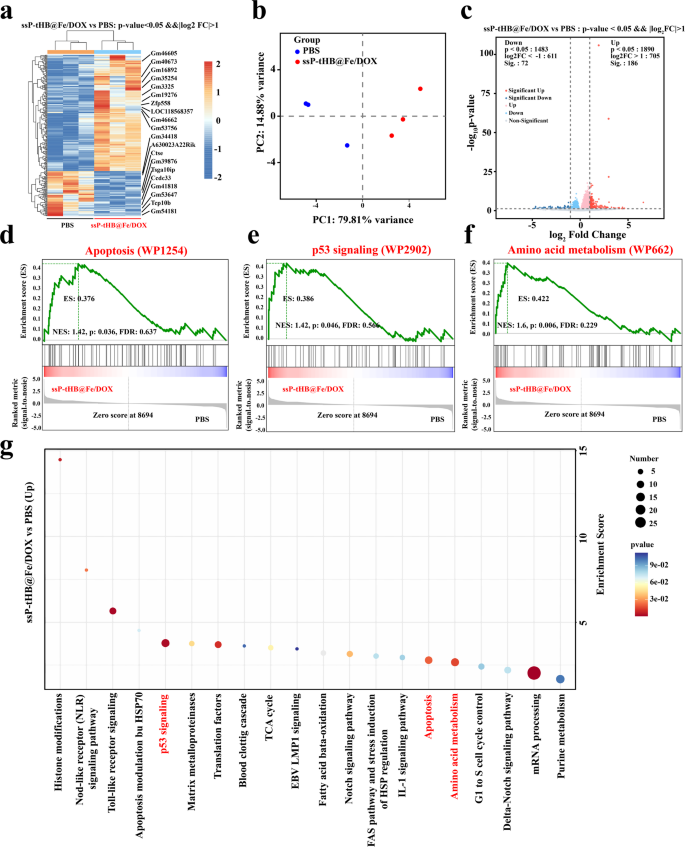
RNA sequencing (RNA-seq) evaluation was performed to guage the therapeutic mechanism of ssP-tHB@Fe/DOX in 4T1 cells. As proven in Fig. 5a, the warmth map of mRNA reveals modifications within the cell transcriptome induced by therapy with ssP-tHB@Fe/DOX. Furthermore, principal element evaluation (PCA) revealed a major clustering relationship between the ssP-tHB@Fe/DOX therapy group and the PBS management group (Fig. 5b). We then performed bioinformatics analyses to match the variations (p-value < 0.05 and|log2 FC| > 1) (Fig. 5c). The outcomes of the gathering discovered that the edge of differentially expressed genes (DEGs) underneath ssP-tHB@Fe/DOX-mediated 4T1 cells was 3373, of which 1873 gene up-regulated, 2101 gene down-regulated. Then, the important thing pathways concerned within the therapeutic impact of ssP-tHB@Fe/DOX had been analyzed via enrichment evaluation. Gene Ontology (GO) enrichment evaluation (Fig. S15) and pathway enrichment evaluation utilizing the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (Fig. 5g) revealed important enrichment in p53 signaling pathways, apoptosis-related processes, and amino acid metabolism. Due to this fact, the obtained outcomes confirmed that therapy with ssP-tHB@Fe/DOX in 4T1 cells may partially exert its anti-cancer impact by activating p53 signaling to induce mobile apoptosis and by modulating amino acid metabolism to induce ferroptosis.
(a) Warmth map of mRNAs associated to therapy development. (b) PCA based mostly on bulk RNA-seq outcomes of the PBS and ssP-tHB@Fe/DOX. (c) Volcano map of the transcriptomic profiles. (d, c, and f) GSEA of apoptosis (WP1254) (d), p53 signaling (WP2902) (e), and amino acid metabolism (WP662) (f) signaling pathway after ssP-tHB@Fe/DOX remedy. (g) Bubble diagram of differentially expressed genes enriched in KEGG
As well as, to higher illustrate the transcriptome modifications induced by ssP-tHB@Fe/DOX, we performed gene set enrichment evaluation (GSEA) on the RNA-seq knowledge. As anticipated, the three considerably enriched signature pathways, similar to apoptosis (WP1254) (Fig. 5d), p53 signaling (WP2902) (Fig. 5e), and amino acid metabolism (WP662) (Fig. 5f), had been displayed. Extra encouragingly, gene regulators concerned in cell apoptosis and ferroptosis mediated by ssP-tHB@Fe/DOX, similar to drivers, suppressors, and markers, had been recognized (Fig. S16, S17, and S18). Finally, we may speculate that breast most cancers might be successfully handled via ssP-tHB@Fe/DOX by inducing each apoptosis and ferroptosis.
Therapeutic effectivity in opposition to 4T1 tumors and biosafety in vivo
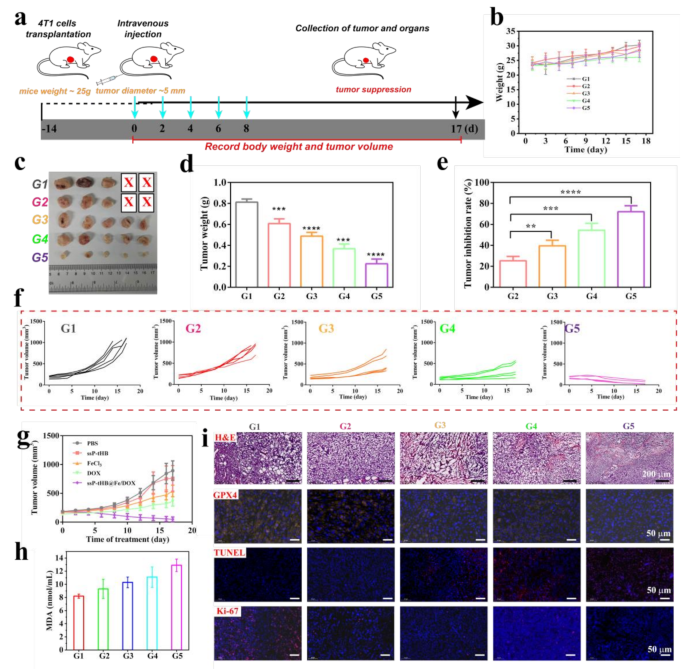
To judge the therapeutic efficacy of the cyclic magnification ferroptosis and DOX-induced chemotherapy in ssP-tHB@Fe/DOX, the therapy’s impact on a subcutaneous 4T1 tumor mannequin was assessed in vivo. Firstly, 4T1 tumor-bearing mice had been efficiently established (roughly tumor quantity: 80 mm3) and had been randomly assigned 5 teams, PBS group (G1), ssP-tHB (G2), FeCl3 (G3), DOX (G4), and ssP-tHB@Fe/DOX (G5, DOX: 6 mg/kg), respectively. Afterwards, we administered the various samples with an equal quantity by way of intravenous injections each two days (5 instances in complete) for a complete of 17 days and recorded the tumor quantity modifications and physique weight fluctuations, as visually depicted in Fig. 6a. All through the therapy interval, 4T1 tumor-bearing mice in all teams confirmed venial upward development in weight (Fig. 6b), suggesting that the therapeutic samples didn’t intrude with regular progress in mice. The tumor progress curves confirmed that, in contrast to the PBS (G1) and ssP-tHB (G2) teams, which exhibited speedy tumor progress, the opposite therapeutic teams (G3, FeCl3; G4, DOX; and G5, ssP-tHB@Fe/DOX) demonstrated important tumor quantity discount. Amongst these, G5 (ssP-tHB@Fe/DOX) was the best, aligning with outcomes from in vitro cell experiments (Fig. 6f and g). Really, this helps the outcomes that ferroptosis-chemotherapeutic mixture remedy is superior to mono-therapy. This statement was additional supported by digital images of the tumors taken 17 days after therapy with totally different samples, as proven in Fig. 6c. At 14 days post-treatment, as proven in Fig. 6d and e, G5 demonstrated probably the most important tumor inhibition as a result of mixed results of ssP-tHB@Fe/DOX-induced environment friendly ferroptosis and DOX-mediated apoptosis.
Anti-tumor therapies with ssP-tHB@Fe/DOX in 4T1 tumor mannequin. (a) Schematic illustration of the in vivo antitumor therapy experiment. (b) Physique-weight modifications of mice (n = 5). (c) Consultant tumor photos of 4T1 tumor-bearing mice receiving varied therapies. (d) Weights of tumors harvested from 4T1 tumor-bearing mice in numerous teams. (e) Tumor progress inhibition charge for every therapy group after 17 days of therapy. (f) Spaghetti curves displaying tumor progress in mice handled in a different way. (j) Tumor progress curves (n = 5) for tumor-bearing mice injected intravenously with G1, G2, G3, G4, and G5, respectively. (h) MDA content material in serum of mice from every therapy group post-treatment. (i) Histological photos of H&E, and TUNEL, antigen Ki-67, GPX4 immunofluorescence-stained tumor slices from the 4T1 tumor-bearing mice at day 17. PBS (G1), ssP-tHB (G2), FeCl3 (G3), DOX (G4), and ssP-tHB@Fe/DOX (G5), **p < 0.01, ***p < 0.001, and ****p < 0.0001
Subsequently, for particular evaluation of pathology, the collected tumors had been stained by way of hematoxylin and eosin (H&E), TdT-mediated dUTP Nick-Finish Labeling (TUNEL), Ki−67, and GPX4 (Fig. 6i). As anticipated, H&E and TUNEL imaging revealed that the ssP-tHB@Fe/DOX group (G5) exhibited a better stage of apoptosis in comparison with the opposite teams. The cell proliferation skill of ssP-tHB@Fe/DOX exhibited a major lower, as indicated by Ki−67 protein staining, in comparison with different therapy teams. These outcomes confirmed that ssP-tHB@Fe/DOX with multi-mode mixture displays important anti-tumor results. Subsequent, we assessed the expression of GPX4 in numerous samples-treated tumors. Comparatively, the expression of GPX4 in tumors was receded by ssP-tHB@Fe/DOX (G5) therapy, proving its skill to boost ferroptosis (Fig. 6i). MDA is pure byproducts of LPO. We measured the MDA expression ranges within the serum of every group after therapy. As introduced in Fig. 6h, in comparison with the PBS group, the expression of MDA was the best within the G5, which once more signifies that ssP-tHB@Fe/DOX may improve LPO and result in ferroptosis.
The biosafety of mice with 4T1 tumors was additionally a vital issue within the therapy course of. Due to this fact, the features of liver (indicators: alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST)) and kidney (indicators: creatinine (CREA), urea (UREA)) had been analyzed, in addition to routine bloods (indicators: platelet hematocrit (PCT), white blood cell (WBC) and pink blood cell (RBC)) of mice had been detected [52]. As proven in Fig. S19, the degrees of ALP, ALT, and AST had been considerably elevated in DOX alone-treated mice (G4), indicating the induction of hepatotoxicity. No important modifications had been noticed within the ranges of ALT, ALP, AST, UREA, CREA, PCT, WBC, and RBC within the G2, G3, and G5 therapy teams. These knowledge indicated no marked poisonous results on the present dose of ssP-tHB@Fe/DOX. Correspondingly, H&E staining outcomes indicated important liver harm completely within the DOX therapy group, whereas no notable accidents had been noticed within the livers, kidneys, or hearts of mice handled with ssP-tHB@Fe/DOX (Fig. S20). These outcomes indicated that ssP-tHB@Fe/DOX had a superb biocompatibility.