Preparation and properties of Fe-Cur CPNs
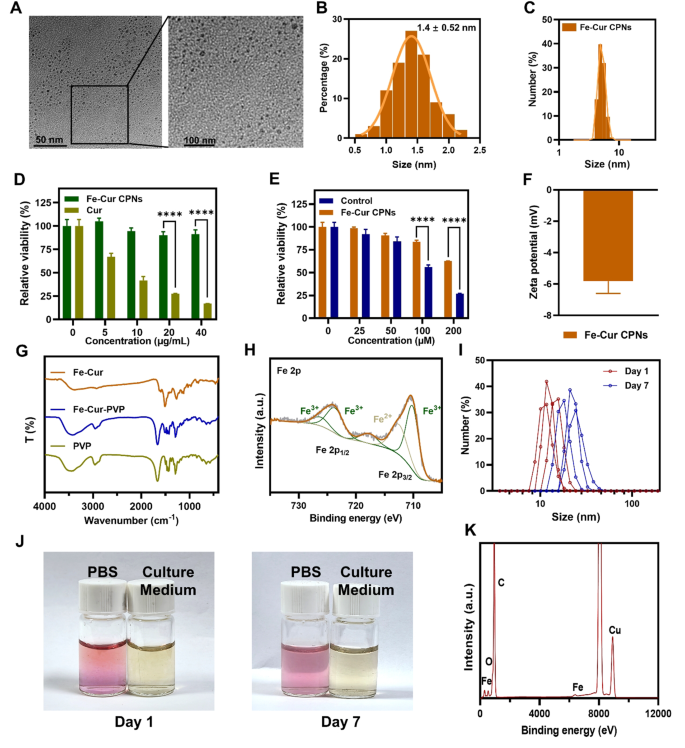
Fe-Cur CPNs have been synthesized through the room temperature stirring technique, which provides ease of operation, managed circumstances, low value, and ease. This compound can cut back ocular irritation, speed up corneal epithelial therapeutic, and inhibit neoangiogenesis. TEM picture revealed that the scale of the Fe-Cur CPNs was roughly 5 nm, and the Fe-Cur CPNs have been distributed abundantly and homogeneously (Fig. 1A). Beneath excessive magnification, the particle measurement distribution evaluation of Fe-Cur CPNs revealed that almost all of particles have been round 4 nm (Fig. 1B), indicating a comparatively uniform measurement distribution. Moreover, the hydrodynamic measurement of the Fe-Cur CPNs, as measured by DLS, corresponded to the scale of the noticed nanodots through TEM (Fig. 1C). In contrast with standard large-size useful supplies, these ultra-small nanodots exhibited exceptional renal metabolism and excretion by means of the urine properties. On this research, Fe-Cur CPN was exactly mixed with PVP to boost the aqueous solubility of Cur. PVP is without doubt one of the most incessantly utilized polymers in pharmaceutical formulations [35]. As a hydrophilic polymer service, PVP possesses distinct benefits owing to its structural composition, characterised by the presence of hydrophobic alkyl and polar amide teams throughout the pyrrolidone ring [36]. This configuration renders it extremely soluble in a various array of natural solvents and drastically enhances its compatibility and complementarity. Quite a few research have underscored its efficacy in rising drug solubility and enhancing stability [37, 38].
The cytocompatibility assay (Fig. 1D and E) revealed that throughout the focus vary of 0–40 µg/mL, the Fe-Cur CPNs group persistently exhibited a excessive cell survival price because the focus elevated. In distinction, cell viability within the Cur-treated group progressively decreased, with Fe-Cur CPNs demonstrating considerably increased relative cell survival than curcumin at concentrations of 20 and 40 µg/mL (P < 0.0001). Constructing on these outcomes, we additional evaluated the viability of the Fe-Cur CPNs at increased concentrations than the untreated controls. Fe-Cur CPNs maintained comparable cell viability to the controls as much as 50 µM; nevertheless, at concentrations of 100 µM and above, a major discount in viability was noticed (P < 0.0001). These findings indicated that the Fe-Cur CPNs exhibit good cytocompatibility at comparatively low concentrations, with a concentration-dependent lower in viability at comparatively excessive concentrations. The zeta potential of the Fe-Cur CPNs was − 5.82 mV (Fig. 1F), indicating their total destructive cost, which mitigated non-specific adsorption to the cell floor and promoted blood circulation. FTIR evaluation offered robust proof for the profitable conjugation of curcumin to PVP. As proven in Fig. 1G, pure PVP displayed a outstanding carbonyl (C = O) stretching peak at 1663 cm⁻1 and a attribute amide II (N–H bending) band at 1494 cm⁻1. Following conjugation, the amide II moved to 1516 cm⁻1, and the C = O peak at 1665 cm⁻1 was noticed in Fe-Cur-PVP. Further C–H stretching vibrations emerged between 2981–2867 cm⁻1, supporting the formation of the PVP–Cur conjugate. Excessive-resolution XPS spectra of Fe–Cur nanoparticles (Fig. 1H) confirmed that quantitative becoming indicated that Fe3⁺ accounted for ~ 75% and Fe²⁺ for ~ 25% of the whole iron content material, confirming that Fe3⁺ is the dominant oxidation state within the coordination advanced, in keeping with curcumin performing as a bidentate ligand. The steadiness check of Fe–Cur CPNs was evaluated below PBS (pH 7.4) and tradition medium circumstances. As proven in Fig. 1J, the colour and readability of the dispersions remained unchanged from Day 1 to Day 7, with no seen aggregation or sedimentation. DLS measurements (Fig. 1I) additional confirmed that the particle measurement distributions remained secure below each circumstances. EDS evaluation (Fig. 1Ok) revealed robust alerts comparable to Fe, C, and O, validating the basic element of the Fe-Cur advanced. These outcomes reveal that Fe-Cur stays intact with out releasing free curcumin, and its organic results come up from the secure metallic–ligand advanced.
Physicochemical characterization, cytocompatibility, and antioxidant properties of Fe-Cur CPNs. (A) TEM photos of Fe-Cur CPNs. (B) Particle measurement distribution of Fe-Cur CPNs. (C) Hydrodynamic diameter of Fe-Cur CPNs measured by DLS. (D, E) Cytocompatibility assay of Fe-Cur CPNs at numerous concentrations. (F) Zeta potential of Fe-Cur CPNs. (G) FTIR spectra of PVP, Fe-Cur and Fe-Cur-PVP conjugates. (H) Excessive-resolution XPS spectra of Fe 2p in Fe-Cur CPNs. (I) DLS profiles of Fe-Cur CPNs after incubation in PBS for 1 and seven days, demonstrating wonderful colloidal stability. (J) Pictures of Fe-Cur CPNs suspensions in PBS and tradition medium at Day 1 and Day 7, displaying no important coloration change or precipitation. (Ok) EDS elemental evaluation of Fe-Cur CPNs
Antioxidant and anti inflammatory results of Fe–Cur CPNs in vitro
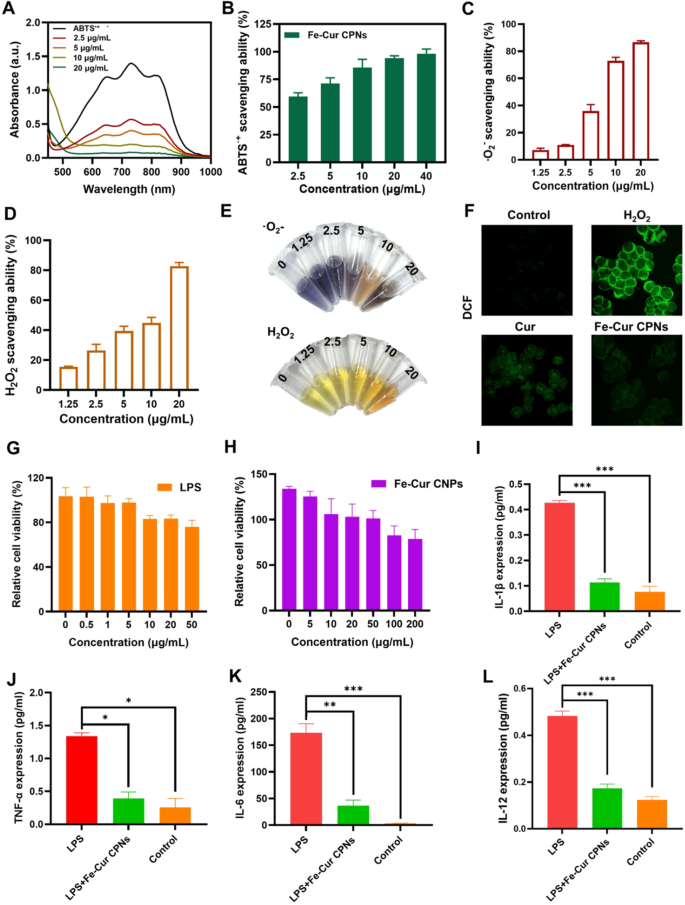
The ABTS scavenging assay is without doubt one of the most prevalent strategies for assessing the antioxidant potential of compounds. As depicted in Fig. 2A, ABTS•+ radicals exhibited a attribute absorption peak at 734 nm, which progressively diminished within the presence of the Fe-Cur CPNs. The outcomes (Fig. 2B) demonstrated that upon the addition of solely 2.5 µg/mL Fe-Cur CPNs, the attribute absorption peak of ABTS•+ considerably weakened, with a corresponding radical scavenging price of 60%. Moreover, the novel scavenging capability of the Fe-Cur CPNs was immediately correlated with their concentrations, because the focus of the Fe-Cur CPNs elevated, as was the novel scavenging price. Notably, at a focus of 20 µg/mL Fe-Cur CPNs, the attribute absorption peak of ABTS•+ almost disappeared, and at 40 µg/mL, the novel scavenging price reached 100%. Along with hydroxyl radical scavenging, Fe–Cur successfully neutralized different reactive oxygen species. As proven in Fig. 2C, superoxide anion (·O2⁻) scavenging elevated dose-dependently from ~ 10% at 1.25 µg/mL to over 85% at 20 µg/mL. Hydrogen peroxide scavenging (Fig. 2D) equally rose from ~ 15% to ~ 80% throughout the identical focus vary. Corresponding colorimetric modifications have been visually obvious in response mixtures (Fig. 2E), the place NBT samples transitioned from deep purple to colorless and Ti(SO4)2 samples modified from yellow to being lined by the colour of the Fe-Cur CPNs. These findings spotlight the strong antioxidant capability of Fe-Cur CPNs throughout a number of ROS varieties. Determine 2F illustrates the evaluation of oxidative harm in rabbit corneal endothelial cells through DCF-DA staining, which reveals ROS ranges by means of fluorescence. The untreated management group exhibited minimal fluorescence, indicating low ROS ranges. In distinction, the H₂O₂-treated group confirmed intense fluorescence, confirming substantial ROS technology and oxidative stress. In contrast with the H₂O₂ group, the Cur-treated group displayed average fluorescence, reflecting a partial discount in ROS ranges and demonstrating the antioxidant impact of Cur. Notably, the Fe-Cur CPNs-treated group exhibited even decrease fluorescence, suggesting a stronger antioxidant impact.
To judge the anti-inflammatory potential of Fe-Cur CPNs, we first established an LPS-induced inflammatory mannequin in HCECs. As proven in Fig. 2G, LPS remedy led to a dose-dependent lower in cell viability. A focus of 20 µg/mL was chosen because the optimum induction dose, because it produced important inflammatory stress with out extreme cytotoxicity. Fe-Cur CPNs displayed negligible cytotoxicity as much as 100 µg/mL, with over 85% cell viability noticed in any respect concentrations examined (Fig. 2H). Primarily based on this, 50 µg/mL was chosen as a consultant therapeutic focus that balances security and potential efficacy for anti-inflammatory analysis. Stimulation with LPS led to a marked enhance within the expression of a number of pro-inflammatory cytokines, together with IL-1β, IL-6, TNF-α, and IL-12, confirming the profitable activation of an inflammatory response in HCECs. Notably, co-treatment with Fe-Cur CPNs considerably attenuated this cytokine surge. IL-1β, an early-phase mediator of innate immune activation, was considerably upregulated following LPS publicity, however Fe-Cur CPNs remedy diminished its ranges to close baseline, akin to the management group (Fig. 2I). The same development was noticed for TNF-α, one other important early pro-inflammatory cytokine, the place Fe-Cur CPNs administration decreased its elevated ranges to almost management ranges (Fig. 2J). IL-6, a central amplifier of the inflammatory cascade, was induced robustly by LPS stimulation, but considerably suppressed by Fe-Cur CPNs co-treatment, although a modest elevation remained in comparison with untreated cells (Fig. 2Ok). Moreover, IL-12, a cytokine implicated in adaptive immune activation and continual irritation, was considerably downregulated by Fe-Cur CPNs remedy to ranges statistically indistinguishable from management (Fig. 2L). These findings collectively reveal that Fe-Cur CPNs exert potent anti-inflammatory results in vitro by concentrating on each early and late cytokine responses, highlighting their therapeutic potential for treating ocular or epithelial inflammatory circumstances.
The NF-κB signaling pathway performs a central function in regulating irritation and oxidative stress [39,40,41,42]. Beneath pathological circumstances resembling tissue harm or an infection, oxidative stress-primarily pushed by the buildup of intracellular ROS-acts as a key upstream activator of the NF-κB pathway. Activation of this classical pathway entails the phosphorylation and subsequent degradation of IκBα, which allows the p65/p50 NF-κB heterodimer to translocate from the cytoplasm to the nucleus. As soon as contained in the nucleus, NF-κB promotes the transcription of assorted pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β) and ROS-generating enzymes (e.g., iNOS, COX-2), thereby establishing a constructive suggestions loop that exacerbates irritation and oxidative harm. Consequently, concentrating on the ROS/NF-κB axis is taken into account a promising technique for mitigating inflammation-associated harm. In vitro experiments utilizing an LPS-induced irritation mannequin in HCECs demonstrated that Fe–Cur CPNs considerably inhibited the expression of key inflammatory cytokines (TNF-α, IL-6, IL-1β, and IL-12) by modulating the NF-κB pathway, whereas concurrently lowering intracellular ROS ranges. These findings recommend that Fe-Cur CPNs exert twin regulatory results on oxidative and inflammatory pathways, underscoring their therapeutic potential for treating corneal harm characterised by extreme irritation and neovascularization.
Antioxidant and anti inflammatory evaluations of Fe-Cur CPNs. (A) UV–vis spectroscopy was used to evaluate the ABTS•+ radical scavenging exercise of Fe-Cur CPNs. (B) ABTS assay was carried out to quantify the novel scavenging potential of Fe-Cur CPNs. (C) NBT discount assay was used to judge superoxide anion (·O2⁻) scavenging exercise. (D) H2O2 scavenging was evaluated through the titanium sulfate assay. (E) Colorimetric NBT and H2O2 assays have been used to visually assess ROS scavenging based mostly on observable coloration modifications. (F) DCF-DA fluorescent probe staining was used to detect intracellular ROS ranges. (G) CCK-8 assay was carried out to judge the impact of LPS on HCECs viability. (H) CCK-8 assay was used to evaluate the cytotoxicity of Fe-Cur CPNs on HCECs. (I–L) ELISA was used to measure the expression ranges of IL-1β (I), TNF-α (J), IL-6 (Ok), and IL-12 (L)
Analysis of ant-CoNV have an effect on
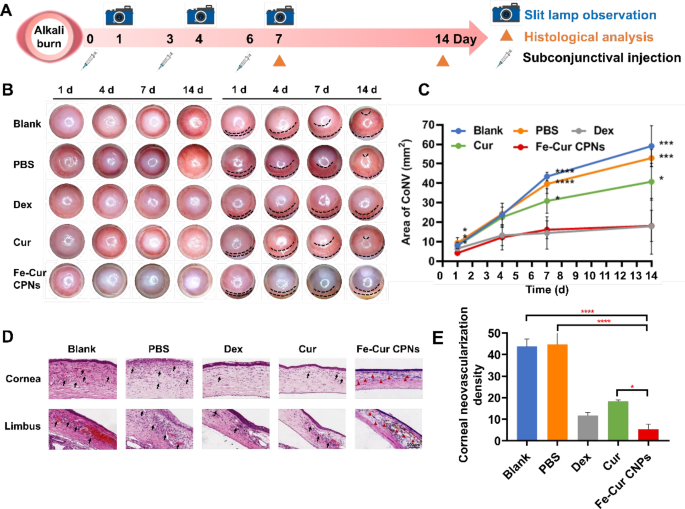
The corneal alkali burn mannequin was created on day 0 and monitored over a 14-day postoperative interval. The expansion of CoNV in every group of rats on days 1, 4, 7, and 14 after corneal alkali burns was noticed below a slit lamp. Some rats have been euthanized on day 7 for in vitro analyses (Fig. 3A). The inhibitory impression of Fe-Cur CPNs on CoNV was evaluated by means of sequential observations, evaluating the proportion of corneal CoNV space between the Fe-Cur CPNs group and different remedy teams (Fig. 3B, C). No remedy was given to the Clean group. From day 1 to day 14 after alkali burns, CoNV quickly accrued from the peripheral a part of the cornea to the cornea. By day 14, CoNV had prolonged to the apex of the cornea, forming an interconnected community overlaying your complete cornea. The expansion of CoNV within the PBS didn’t considerably differ from that within the Clean group, indicating that subconjunctival injection remedy had no important impact on the experiment. As compared with the fast and vigorous progress of CoNV within the first two teams, the expansion of CoNV within the Fe-Cur CPNs group was considerably slowed down, with a drastically diminished extent of corneal involvement. By the 14th post-injury day, solely the peripheral a part of the cornea was concerned, and the world of CoNV was considerably decrease than that of the Clean and PBS teams on the aforementioned time factors, with statistically important variations on the first (P < 0.05), seventh (P < 0.0001), and 14th (P < 0.001) post-injury days (Fig. 3C). Within the Cur group, the CoNV space was smaller than within the Clean and PBS teams; nevertheless, it remained considerably larger than that within the Fe-Cur CPNs group at numerous time factors (Days 1, 7, and 14), with notable statistical variations (P < 0.05). These outcomes confirmed that topical remedy with Cur after corneal alkali burns might inhibit the expansion of corneal neovascularization. Nevertheless, since pure Cur was not modified in any approach, its poor bioavailability resulted in its anti-angiogenic impact not being absolutely exerted in vivo. On this research, Fe-Cur CPNs drastically elevated the water solubility of pure Cur and improved its bioavailability, thereby considerably enhancing its bioactivity in vivo. In the meantime, Dex is a traditional therapeutic drug for the remedy of CoNV in scientific settings, identified for its affordability and efficacy. On this experiment, the inhibitory impact of Fe-Cur CPNs on CoNV was akin to that of the Dex group, with no important distinction within the space of CoNV noticed at any time level (P > 0.05). This offered adequate proof that the impact of Fe-Cur CPNs in inhibiting corneal neovascularization after alkali burns was wonderful and will attain scientific remedy ranges.
Determine 3D presents the pathological modifications noticed within the corneal limbus for every group. Within the Clean group, the corneal limbus displayed heightened blood stream, characterised by quite a few neovascular lumens within the stromal layer that prolonged into the cornea, together with pronounced aggregation of inflammatory cells. This sample was additionally noticed within the PBS group. In distinction, the Cur group exhibited a extra orderly construction of the corneal limbus, characterised by fewer neovascular lumens infiltrating the corneal stroma and diminished inflammatory cell infiltration. Within the Dex group, corneal limbus blood stream was considerably diminished in comparison with the previous teams, with solely a restricted variety of neovascular lumens penetrating the corneal stroma. No obvious neovascularization was noticed within the corneal limbus stroma of the Fe-Cur CPNs group, the place a considerable quantity of brownish-yellow Fe-Cur CPNs particles progressively infiltrated the cornea from the limbus into the stroma. The neovascularization space ratio was quantified in additional element, as displayed in Fig. 3E. On day 7, the Fe-Cur CPNs group confirmed the smallest CoNV space at 5.33 ± 2.31, considerably lower than the Clean group (43.67 ± 3.51) and the PBS group (44.67 ± 7.77) (P < 0.0001), in addition to the Cur group (18.33 ± 0.58) (P < 0.05), and akin to the Dex group (11.67 ± 1.53) (P > 0.05). These findings align with the noticed traits in corneal histopathological modifications throughout the teams, confirming the initiation of CoNV from the corneal limbus and its gradual extension into the cornea. Notably, there was no evident neovascularization noticed within the corneal rim or cornea of the Fe-Cur CPNs group, implying that the event of CoNV might have been inhibited at its supply. This advised that Fe-Cur CPNs have been simpler in inhibiting CoNV in comparison with different remedies.
Fe-Cur CPNs inhibit corneal neovascularization. (A) Timeline of examinations following a corneal alkali burn. (B) Corneal neovascularization was assessed at 1, 4, 7, and 14 days post-burn. (C) The quantified corneal neovascularization inside 14 days. (D) Pathohistological and histological modifications in corneal and limbal neovascularization within the rat cornea for every group on day 7 post-burn harm. (E) Statistical evaluation outcomes of CoNV density for every group on day 7 post-burn harm
Analysis of epithelial restore and corneal permeability of Fe–Cur CPNs
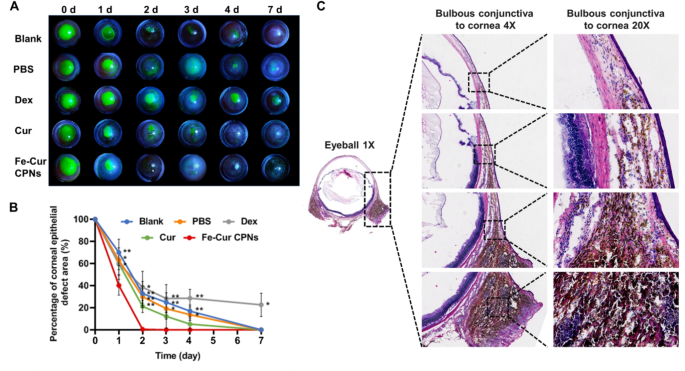
The restore of corneal epithelial defects in every group of rats with corneal alkali burns was monitored utilizing sodium fluorescein staining below a slit lamp on day 0 (instantly post-burn) and on days 1, 2, 3, 4, and seven (Fig. 4A). The therapeutic price of corneal epithelium in numerous remedy teams was in contrast (Fig. 4B). On this experiment, following corneal alkali burn, the corneal epithelium was almost utterly indifferent in all rat teams. Regeneration of the corneal epithelium was achieved inside 7 days in each the Clean and PBS teams, in keeping with the time-frame for corneal epithelial regeneration in third-degree alkali burns. Conversely, the Fe-Cur CPNs group started re-epithelialization on the primary day after the burn, with the corneal epithelium repairing considerably sooner than within the Clean group (P < 0.01), PBS group (P < 0.05), and Dex group (P < 0.05). On day 2, the Fe-Cur CPNs group confirmed a considerably accelerated epithelial regeneration price in comparison with the Clean, PBS, and Cur teams (P < 0.01), in addition to a notably sooner price than the Dex group (P < 0.05). On days 3 and 4, the Fe-Cur CPNs group demonstrated considerably sooner corneal epithelial therapeutic in comparison with the clean (P < 0.01), PBS (P < 0.05), and Dex (P < 0.01) teams. By day 7, epithelial restore was almost full within the Fe-Cur CPNs group, with therapeutic occurring considerably sooner than within the Dex group (P < 0.05). In distinction, central corneal epithelial restore remained incomplete by day 7 post-injury, underscoring the inhibitory impact of Dex on corneal re-epithelialization in alkali burn remedy. Furthermore, such remedy can doubtlessly induce adversarial results, together with corneal stromal lysis, ulceration, and perforation, whereas inappropriate utilization might compromise native tissue resistance, precipitating circumstances resembling glaucoma and cataracts [43, 44]. Therefore, the utilization of Fe-Cur CPNs within the administration of corneal alkali burns facilitates the early regeneration of corneal epithelium, mitigating additional deterioration of the corneal stroma and intraocular tissues.
Total, Fe-Cur CPNs remedy successfully suppressed the inflammatory response, promoted corneal epithelial regeneration, and considerably enhanced tissue restore. As depicted in Fig. 4C, on the seventh day post-alkali burn, a considerable quantity of Fe-Cur CPNs answer continued within the bulbar conjunctiva bilaterally, diffusing all through your complete bulbar conjunctival tissue area as noticed in the entire eye through Hematoxylin and Eosin (HE) staining of rat corneas within the Fe-Cur CPNs group. Moreover, the drug exhibited penetration and diffusion in direction of the corneal middle within the HE sections, with a dispersed distribution of the Fe-Cur CPNs answer evident on the corneal conjunctival junction, and a notable accumulation of the drug noticed within the stromal layer of the corneal limbus. Within the alkali burn mannequin, CoNV initiation exactly focused the cornea from the corneal limbus, contrasted with the Clean and PBS teams, which exhibited larger neovascularization and enhanced blood stream into the cornea. Fe-Cur CPNs exhibited steady penetration and diffusion into the peripheral stroma through the corneal limbus, enabling steady inhibition of CoNV technology.
Analysis of Epithelial Restore and Corneal Permeability of Fe–Cur CPNs. (A) Commentary of corneal epithelial sodium fluorescein-stained areas was performed in every group on days 0, 1, 2, 3, 4, and seven following corneal alkali burns. (B) Semi-quantitative evaluation of the proportion space of corneal epithelial defects in every group at numerous time factors following corneal alkali burns. (C) Permeability of Fe-Cur CPNs from the bulbar conjunctiva to the cornea, noticed on day 7 post-alkali burn
Anti-angiogenic results of Fe-Cur CPNs
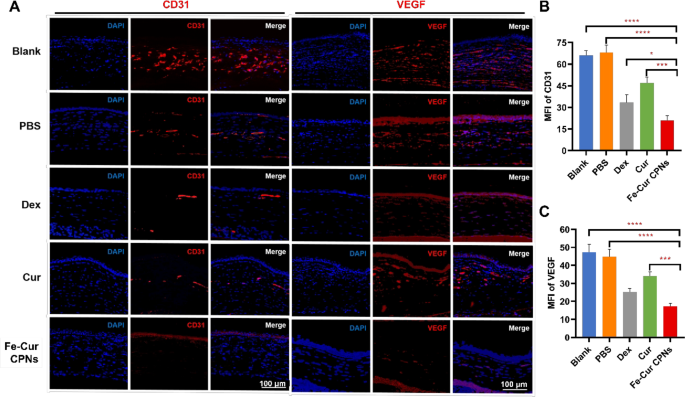
On day 7 post-alkali burn, the expression ranges of key angiogenic markers, together with CD31 and VEGF, within the corneal stroma diversified considerably among the many remedy teams. VEGF, an important regulator of angiogenesis, is often upregulated in inflammatory and hypoxic environments, whereas CD31 serves as an endothelial cell marker indicating the formation of CoNV [45]. Fe-Cur CPNs remedy resulted in a marked discount within the expression of each markers, indicating a potent anti-angiogenic impact. Within the clean and PBS teams, elevated ranges of those markers have been noticed, with minimal variations between the 2. Nevertheless, remedies with Dex, Cur, and particularly Fe-Cur CPNs, resulted in substantial reductions in CD31 and VEGF expression, with Fe-Cur CPNs displaying essentially the most pronounced impact (Fig. 5A). As proven in Fig. 5B, the MFI of CD31 within the Fe-Cur CPNs group was notably decrease than in all different teams, with the bottom recorded MFI worth (20.94 ± 3.21). This was adopted by the Dex group (33.41 ± 5.46) (P < 0.05), whereas the Cur group exhibited a average lower (46.99 ± 3.92) (P < 0.001). The Clean and PBS teams had the very best MFI values (66.19 ± 3.12 and 68.01 ± 5.17, respectively) (P < 0.0001). By way of VEGF expression, Fig. 5C additional supported these findings. Each the Clean and PBS teams confirmed plentiful VEGF-positive staining, with MFI values of 47.38 ± 4.25 and 44.93 ± 4.11, respectively. Within the Cur group, VEGF expression was primarily situated within the CoNV lumen, with an MFI worth of 34.01 ± 2.31. The Dex group exhibited weak VEGF expression within the stromal layer, with diffuse staining and an MFI worth of 25.16 ± 1.99. Within the Fe-Cur CPNs group, VEGF-positive staining within the corneal stromal layer was considerably diminished, with the bottom VEGF expression noticed (MFI worth of 17.17 ± 1.77). The variations in MFI between the Fe-Cur CPNs group and the Clean and PBS teams (P < 0.0001) in addition to the Cur group (P < 0.001) have been statistically important, indicating a powerful anti-angiogenic impact of Fe-Cur CPNs by lowering VEGF expression. The above outcomes spotlight the efficacy of Fe-Cur CPNs in inhibiting corneal neovascularisation by concentrating on and lowering the expression of CD31 and VEGF.
Antioxidative and anti-Inflammatory results of Fe-Cur CPNs
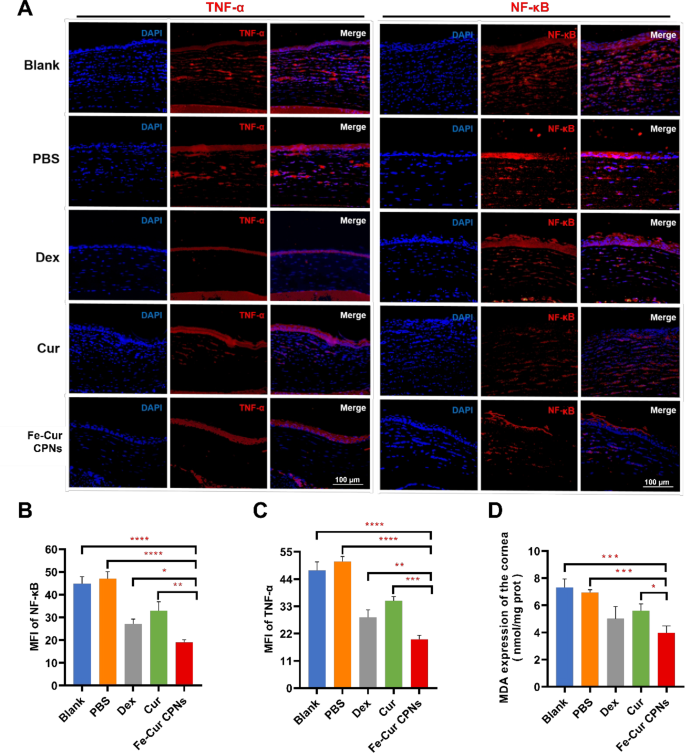
Along with their anti-angiogenic results, Fe-Cur CPNs reveal important antioxidant and anti inflammatory properties. Following alkali burns, the extent of ROS in corneal tissue rises sharply, resulting in oxidative stress and mobile harm [46]. ROS trigger direct mobile harm and likewise activate NF-κB, which strikes into the nucleus to upregulate inflammatory elements like IL-1β and TNF-α [47]. These inflammatory mediators exacerbate the corneal inflammatory response by recruiting neutrophils and selling VEGF manufacturing, thereby making a vicious cycle of irritation and angiogenesis [48]. Determine 6A demonstrates the inhibitory impression of Fe-Cur CPNs on TNF-α and NF-κB expression in corneal tissue, as evaluated by means of immunofluorescence staining. Compared to the Clean and PBS teams, the pink fluorescence depth of TNF-α and NF-κB within the Fe-Cur CPNs group was considerably diminished. Furthermore, the staining ranges within the Fe-Cur CPNs group have been additionally decrease than these noticed within the Dex and Cur remedy teams, indicating a extra pronounced inhibitory impact. Therapy with Fe-Cur CPNs considerably decreased these inflammatory markers. Particularly, the NF-κB degree with an MFI of 19.11 ± 1.01 and the TNF-α degree of 19.74 ± 1.55 within the Fe-Cur CPNs group have been considerably decrease than these within the Clean and PBS teams (P < 0.0001). In comparison with Cur and Dex alone, Fe-Cur CPNs demonstrated superior efficacy in inhibiting inflammatory elements induced by alkali burns. The NF-κB degree with an MFI of 32.86 ± 4.04 and the TNF-α degree of 35.25 ± 1.7 within the Cur group have been considerably increased than these within the Fe-Cur CPNs group (P < 0.01 and P < 0.001, respectively). The Dex group exhibited decrease ranges of NF-κB (MFI of 27.19 ± 2.09) and TNF-α (28.59 ± 3.01) in comparison with the Cur group; nevertheless, these ranges remained considerably elevated relative to the Fe-Cur CPNs group (P < 0.05 and P < 0.01, respectively) (Fig. 6B and C). The antioxidant efficacy of Fe-Cur CPNs was additionally evident within the important discount of the oxidative stress marker MDA. The MDA degree within the Fe-Cur CPNs group (3.97 ± 0.52 nmol/mg) was considerably diminished in comparison with the Clean group (7.30 ± 0.64 nmol/mg) and the PBS group (6.97 ± 0.19 nmol/mg) (P < 0.001). Though the Cur group additionally exhibited a decrease MDA degree (5.60 ± 0.51), it remained considerably increased than that of the Fe-Cur CPNs group (P < 0.05) (Fig. 6D). The immunofluorescence photos additional confirmed that Fe-Cur CPNs successfully suppressed corneal irritation and mitigated oxidative harm by scavenging ROS, inhibiting NF-κB activation, and lowering TNF-α expression. These findings underscored the superior antioxidant and anti inflammatory capabilities of Fe-Cur CPNs in comparison with Cur alone, contributing to diminished corneal neovascularization and enhanced corneal therapeutic.
Earlier research primarily targeted on the systemic anti-inflammatory potential of Fe-Cur coordination complexes, demonstrating their potential to modulate inflammatory cytokines in macrophages [49, 50], most cancers cells [51], or systemic illness fashions [52, 53]. These works typically emphasised the chemical design or intracellular supply properties of the complexes. In distinction, our research targets a distinct therapeutic state of affairs—corneal alkali burn-induced neovascularization, which presents distinctive challenges in drug supply as a result of ocular floor barrier and the necessity for localized, sustained anti-inflammatory motion. Due to this fact, our emphasis will not be solely on the anti-inflammatory mechanism itself, however extra importantly on find out how to successfully ship curcumin to the ocular tissue utilizing Fe coordination chemistry. To this finish, we developed ultrasmall Fe-Cur nanoparticles (< 10 nm) appropriate for subconjunctival injection, a supply technique that permits enhanced corneal penetration, extended retention, and localized therapeutic efficacy. We additional demonstrated, each in vivo and in vitro, that our Fe-Cur NPs considerably diminished inflammatory cytokine ranges (TNF-α, IL-6, IL-1β) and ROS manufacturing, whereas additionally suppressing NF-κB expression in corneal tissue, with out inflicting native or systemic toxicity. These findings present application-specific proof that distinguishes our system from beforehand reported Fe-Cur formulations and helps its potential as a focused nanomedicine for ocular irritation and neovascularization. Along with its well-established anti-inflammatory and antioxidant actions, curcumin has additionally been reported to own potent antibacterial and anti-infective properties, which can present added therapeutic worth within the remedy of corneal alkali burns. A number of research have demonstrated that curcumin reveals broad-spectrum antimicrobial exercise, significantly towards widespread ocular pathogens resembling Staphylococcus aureus and Pseudomonas aeruginosa [20]. The underlying mechanisms embrace disruption of bacterial cell membranes, inhibition of quorum sensing pathways, and suppression of bacterial virulence gene expression, finally resulting in impaired bacterial progress and biofilm formation. Contemplating that an infection is a significant complication in alkali-induced corneal accidents, the intrinsic antibacterial properties of Fe–Cur CPNs might contribute to stopping secondary infections and supporting tissue restoration. Though antibacterial exercise was in a roundabout way assessed on this research, this potential impact warrants additional investigation and will characterize an extra therapeutic mechanism of Fe–Cur CPNs.
Security analysis
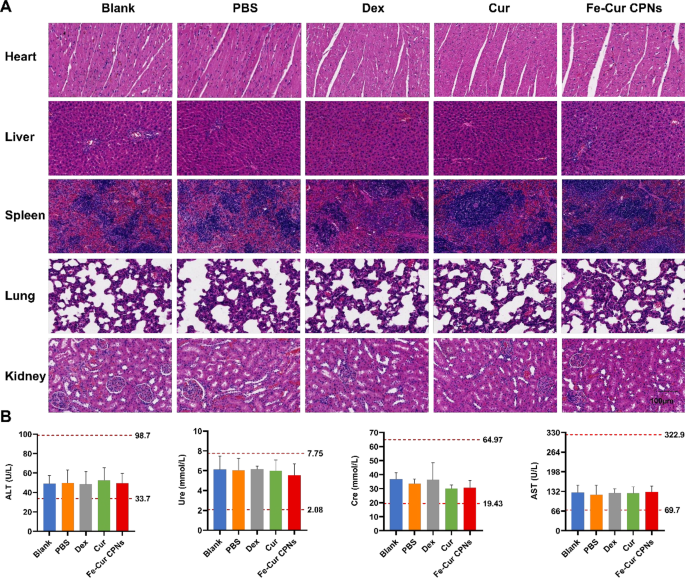
We examined the pathological sections of the center, liver, spleen, lungs, and kidneys from every group on the seventh day following corneal alkali burns (Fig. 7A). Liver cells in all teams confirmed no indicators of injury after drug remedy, and kidney excretion remained regular. Moreover, the center, lungs, and different main organs displayed regular morphology and performance. Moreover, we assessed the blood biochemical liver perform indexes (ALT and AST) and the kidney perform indexes (Ure and Cre) (Fig. 7B). The values for the 4 indices in all teams have been throughout the regular vary, with no important variations between the teams (P > 0.05). These outcomes indicated that corneal alkali burns didn’t induce important harm to the primary organs of the physique. These findings advised that the Fe-Cur CPNs, akin to different therapeutic medicine, didn’t entail any apparent poisonous unintended effects, demonstrating comparatively good drug security.
Cur, acknowledged as a protected compound, has obtained the designation of ‘Typically Acknowledged as Secure’ (GRAS) from the US Meals and Drug Administration (US FDA). The modified curcumin nanocomponent, PVP, is a non-toxic and non-carcinogenic substance with antitoxic properties, assuaging the dangerous results of medicine and different compounds on the physique. Chen et al. [54]. synthesized tannic acid-bortezomib (BTZ) nanoparticles encapsulated in PVP shells. Cytotoxicity examination revealed that changed BTZ nanoparticles exhibited decreased cytotoxicity in comparison with BTZ nanoparticles alone. This research employed the therapeutic mode of bulbar subconjunctival injection, enabling a number of administrations of the drug beneath the conjunctiva with extended motion, thus circumventing drawbacks resembling ocular floor irritation signs ensuing from eye drop dispensation and frequent drug administration.