Analysis of collagen degradation in atherosclerosis
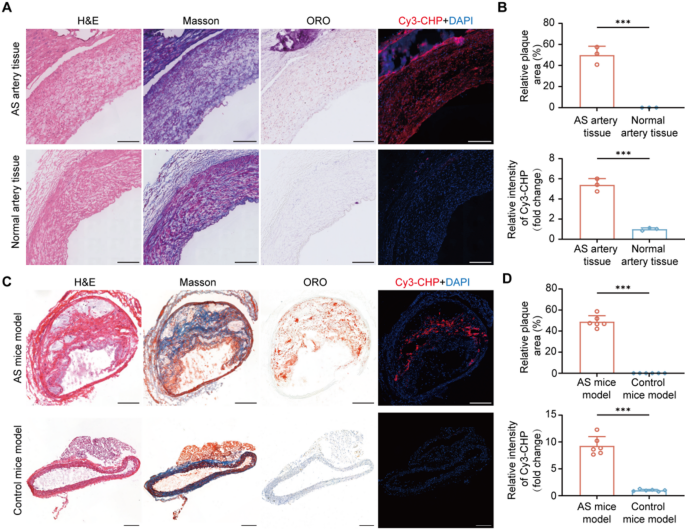
To analyze the in vivo tissue selectivity of CHP within the atherosclerosis, we carried out histological staining and fluorescence staining on each human artery tissues and atherosclerosis mice fashions. As proven in Fig. 1A, histological staining confirmed the presence of atherosclerotic plaques in arteries from AS sufferers, whereas regular arteries exhibited no important lipid accumulation. In response, fluorescence staining demonstrated sturdy Cy3-CHP indicators in atherosclerotic plaques with roughly a 5.38 fold improve in fluorescence depth in comparison with regular artery tissues (AS arteries vs. Regular arteries, 5.38 ± 0.63 vs. 1.00 ± 0.14, n = 3, P < 0.001) (Fig. 1B), indicating the presence of degraded collagen. Equally, within the atherosclerotic mouse mannequin, histological staining additional validated the formation of atherosclerotic plaque (Fig. 1C). Fluorescence staining outcomes confirmed a 9.27 fold improve in Cy3-CHP fluorescence depth in AS mice mannequin in comparison with management mice mannequin (AS mice vs. Management mice, 9.27 ± 1.72 vs. 1.00 ± 0.18, n = 6, P < 0.001) (Fig. 1D), additional supporting the selective binding of CHP to the degraded collagen in atherosclerotic plaques. These findings indicated that collagen degradation have been considerably enhanced in atherosclerotic plaques. As a peptide probe particularly designed to bind degraded collagen, CHP successfully highlighted the collagen degradation in atherosclerotic plaque. Due to this fact, CHP held potential as a molecular probe for focused imaging and remedy of atherosclerotic plaques.
A) Consultant H&E, ORO, Masson, and fluorescence staining photos of atherosclerotic human artery tissues and regular artery tissues. B) Quantitative evaluation of relative plaque space within the aorta and the relative depth of Cy3-CHP fluorescence in human artery tissues. C) Staining and fluorescence photos of atherosclerotic animal mannequin in comparison with management mice mannequin. D) Quantitative evaluation of relative plaque space and Cy3-CHP fluorescence depth in mice fashions. Experimental information have been expressed as imply ± SD. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance. The size bars have been 200 μm
Preparation of the molecular imaging nanoparticles
CHP, functionalized with an azide group on the finish of its peptide chain, was utilized to focus on atherosclerotic plaques. DBCO-NHS ester served as a linker within the copper-free click on chemical response, which was used to conjugate CHP to ICG@BSA imaging NPs. This course of resulted within the formation of a photoacoustic imaging probe with CHP because the focusing on moiety, ICG for photoacoustic imaging, and BSA because the nanocarrier.
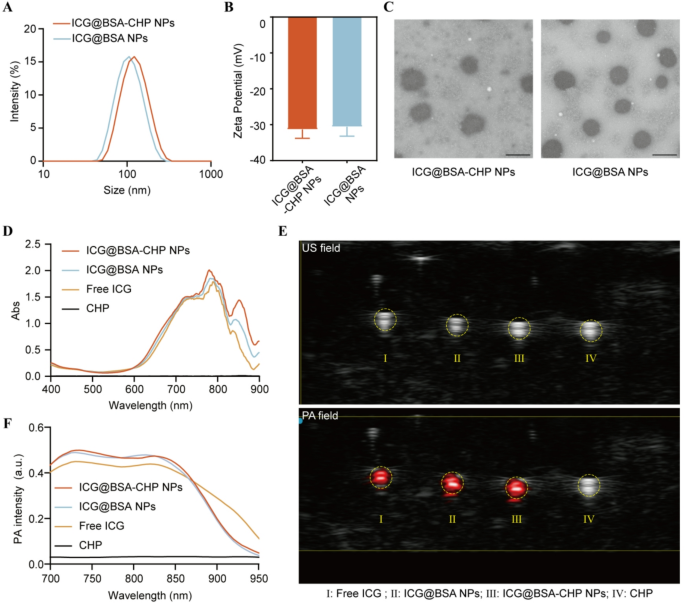
The particle measurement of ICG@BSA-CHP NPs and ICG@BSA NPs have been decided by DLS as 117 nm (polydispersity index, PDI = 0.105) and 105 nm (PDI = 0.115), respectively. The dimensions distribution curves have been offered in Fig. 2A. In the meantime, the zeta potentials of ICG@BSA-CHP NPs and ICG@BSA NPs have been measured at −31.3 mV and − 30.6 mV, respectively (Fig. 2B). Moreover, transmission electron microscopy (TEM) photos confirmed that each nanoparticle varieties possess uniform, spherical shapes (Fig. 2C).
Ultraviolet-visible (UV-Vis) absorption spectroscopy demonstrated the profitable loading of ICG throughout the albumin NPs, no important alterations within the absorption profile upon CHP conjugation (Fig. 2D).
To additional notice the in vivo imaging of atherosclerotic plaques, PA imaging was carried out. Nanoparticles containing ICG can produce PA indicators underneath pulsed laser irradiation by changing laser power into warmth. Accordingly, PA photos of nanoparticles inside a tube have been acquired, and the corresponding PA spectra have been recorded. Determine 2E demonstrated that each ICG@BSA-CHP NPs and ICG@BSA NPs exhibited sturdy PA indicators in comparison with CHP options alone. Just like the UV-Vis absorption outcomes, conjugating CHP had no important impact on the PA spectra of ICG@BSA NPs (Fig. 2F). The obtained spectra function reference signatures for figuring out these nanoparticles in subsequent in vivo PA imaging research.
Characterizations of PA imaging albumin NPs. A) Particle Measurement distribution and B) Zeta potential of ICG@BSA-CHP NPs and ICG@BSA NPs. C) Morphology of NPs noticed by TEM. The size bars have been 100 nm. D) UV-vis absorption spectroscopy of ICG@BSA-CHP NPs and ICG@BSA NPs. E) Ultrasonic (US) and PA picture of free ICG, ICG@BSA NPs, ICG@BSA-CHP NPs and CHP answer. F) In vitro photoacoustic spectra of free ICG, ICG@BSA NPs, ICG@BSA-CHP NPs and CHP answer at completely different wavelengths
Biosafety analysis of ICG@BSA-CHP NPs
To judge the biodistribution and potential toxicity of ICG@BSA-CHP NPs, we carried out fluorescence (FL) photos of ex vivo most important organs from ApoE-/- mice at 1, 6, 12, and 24 h following intravenous injection of the NPs. The FL photos revealed that after injection, the ICG@BSA-CHP NPs initially confirmed sturdy fluorescence indicators, significantly within the liver and kidneys. Over time, these indicators diminished as a consequence of excretion through hepatic metabolism, with fluorescence nearly solely cleared inside 24 h (Determine S1A and B). These findings prompt that the ICG@BSA-CHP NPs have been effectively cleared from the physique inside a comparatively quick time, minimizing the chance of long-term toxicity.
Moreover, we carried out hematological evaluation and H&E staining of the primary organs (coronary heart, liver, spleen, lung and kidney) from the ApoE-/- mice at 1 day, 7 days and 14 days after the intravenous injection of the ICG@BSA-CHP NPs. As listed in Determine S1C, no important adjustments of the hemocyte check and features of liver and kidneys have been noticed after injection. Furthermore, the H&E staining outcomes demonstrated that the tissues maintained regular buildings and no apparent organ injury or inflammatory lesions have been noticed after injection (Determine S1D). Total, these outcomes collectively indicated that ICG@BSA-CHP NPs exhibited the nice biosafety profiles in ApoE-/- mice, supporting their potential for additional in vivo experiments.
In vivo PA imaging and ex vivo FL imaging
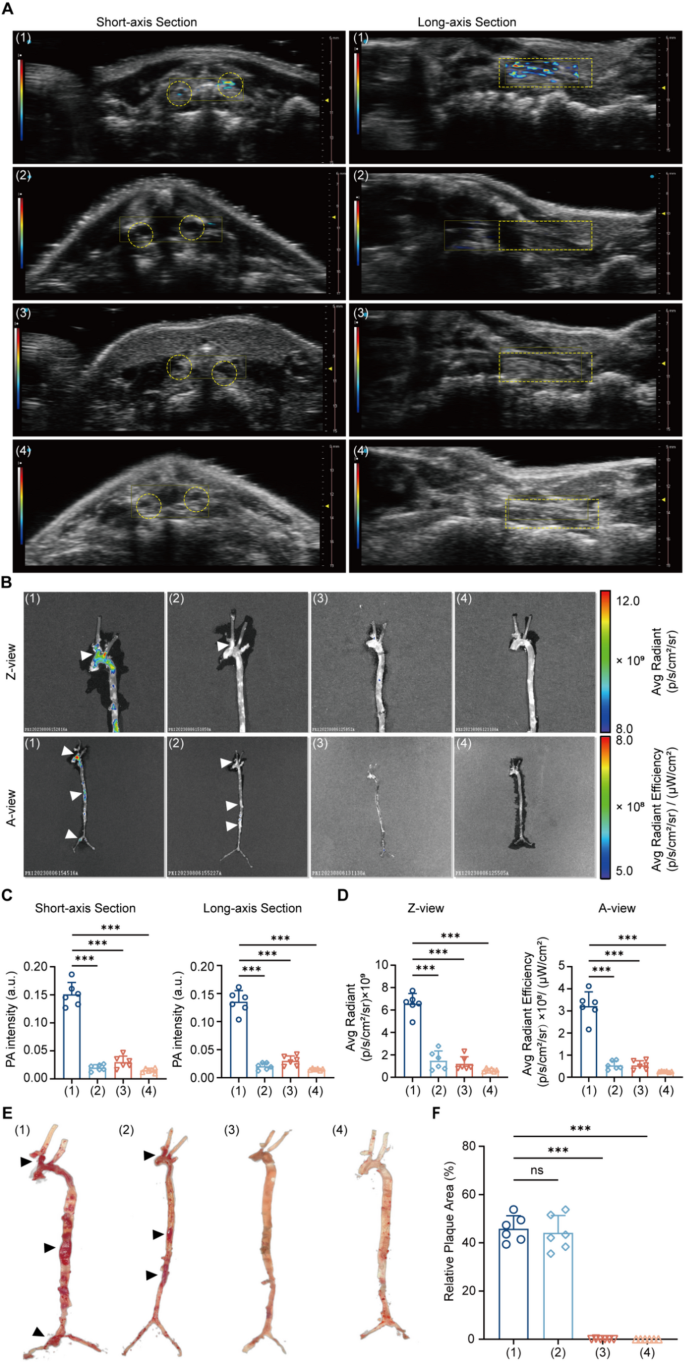
After establishing the PA NPs, atherosclerotic mice have been anesthetized and subjected to PA imaging for in vivo atherosclerotic plaque imaging. Contemplating occlusion avoiding and multidimensional analysis, we collected ultrasonic (US) and PA photos from each short-axis and long-axis views (Fig. 3A).
Upon intravenous injection of ICG@BSA-CHP NPs, the carotid arteries of atherosclerotic (AS) mice mannequin exhibited a considerably stronger PA sign in comparison with management mice, each within the quick axis and lengthy axis views (quick axis: AS mice vs. Management mice, 0.15 ± 0.02 vs. 0.03 ± 0.01, n = 6, P < 0.001; lengthy axis: AS mice vs. Management mice, 0.14 ± 0.02 vs. 0.03 ± 0.01, n = 6, P < 0.001). This enhanced PA sign indicated that ICG@BSA-CHP NPs successfully differentiate between atherosclerotic and regular arteries, possible as a consequence of their focusing on skill for atherosclerotic plaques. Moreover, when evaluating AS mice mannequin injected with ICG@BSA-CHP NPs to these injected with ICG@BSA NPs, the previous confirmed a markedly larger PA sign (quick axis: ICG@BSA-CHP NPs vs. ICG@BSA NPs, 0.15 ± 0.02 vs. 0.02 ± 0.01, n = 6, P < 0.001; lengthy axis: AS mice vs. Management mice, 0.14 ± 0.02 vs. 0.02 ± 0.01, n = 6, P < 0.001). This end result indicated that the incorporation of CHP into the nanoparticles improved focusing on and recognition of atherosclerotic plaques (Fig. 3C).
To additional validate the specificity of PA indicators for atherosclerotic plaques, ex vivo FL was carried out on remoted aorta and carotid arteries, adopted by ORO staining. In step with PA imaging outcomes, AS mice mannequin injected with ICG@BSA-CHP NPs confirmed considerably larger FL indicators in comparison with different teams in carotid arteries (A view : AS mice + ICG@BSA-CHP NPs vs. AS mice + ICG@BSA NPs vs. Management mice + ICG@BSA-CHP NPs vs. Management mice + ICG@BSA NPs, 3.22 ± 0.64 vs. 0.54 ± 0.19 vs. 0.54 ± 0.20 vs. 0.26 ± 0.24, n = 6, P < 0.001; Z view : AS mice + ICG@BSA-CHP NPs vs. AS mice + ICG@BSA NPs vs. Management mice + ICG@BSA-CHP NPs vs. Management mice + ICG@BSA NPs, 6.50 ± 0.92 vs. 1.50 ± 0.85 vs. 1.22 ± 0.62 vs. 0.64 ± 0.14, n = 6, P < 0.001) (Fig. 3B and D). This enhanced fluorescence indicated that CHP enhances nanoparticle enrichment in atherosclerotic plaque areas as a consequence of its focusing on functionality.
Moreover, the alignment between FL imaging and ORO staining revealed that the fluorescence accumulation areas (white arrowheads) intently matched the dye accumulation areas noticed within the ORO staining photos (black arrowheads) within the carotid artery, aortic arch, and thoraco-abdominal aortic junction. This correspondence additional indicated that ICG@BSA-CHP NPs successfully realized atherosclerotic plaques in vivo and in vitro (Fig. 3B and F).
In vivo and Ex vivo Imaging. Experimental mice have been divided into 4 teams based on completely different therapies: (1) AS mice mannequin intravenously injected with ICG@BSA-CHP NPs; (2) AS mice mannequin intravenously injected with ICG@BSA NPs; (3) Management mice mannequin intravenously injected with ICG@BSA-CHP NPs; (4) Management mice mannequin intravenously injected with ICG@BSA NPs. A) In vivo PA photos of each short- and long-axis sections of the carotid artery for every group. Carotid arteries indicated by yellow circle (short-axis part) and rectangle (long-axis part). B) Ex vivo fluorescence imaging of the harvested aorta and carotid artery have been captured in Z-view and A-view. Vital areas of atherosclerotic plaque indicated by white arrowheads. Quantitative statistics of in vivo PA depth C) and ex vivo FL depth D) of various teams. E) ORO staining images of the aorta and carotid arteries of the experimental mice, with important atherosclerotic plaque areas confirmed by black arrowheads. F) Quantitative statistics of plaque areas within the completely different teams. Information in (C, D, F) have been expressed as imply ± SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance
Preparation of therapeutic NPs
In alignment with the preparation of PA NPs, we used PTX@HSA NPs as the first therapeutic agent. CHP was then conjugated to the PTX@HSA NPs through a copper-free click on chemical response as described beforehand.
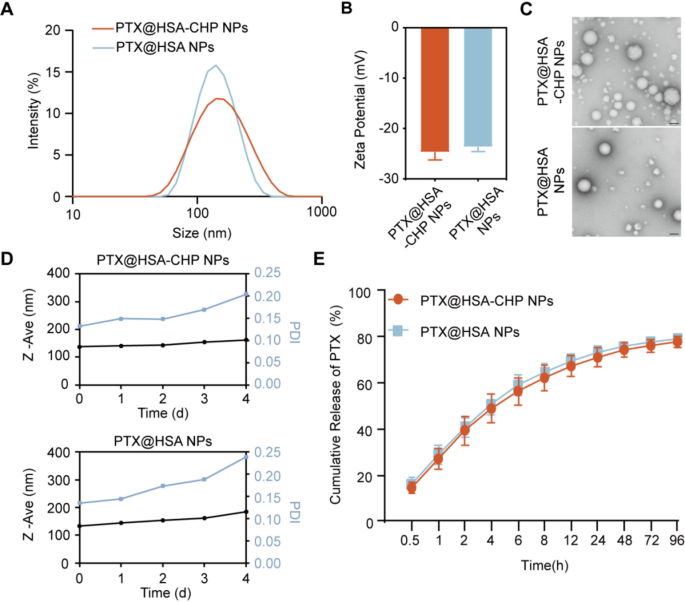
The particle sizes of PTX@HSA-CHP NPs and PTX@HSA NPs have been measured utilizing DLS, revealing sizes of 136.7 nm (PDI = 0.192) and 133.1 nm (PDI = 0.125), respectively (Fig. 4A). The zeta potentials of PTX@HSA-CHP NPs and PTX@HSA NPs have been − 24.67 mV and − 23.57 mV, respectively (Fig. 4B). Transmission electron microscopy (TEM) photos confirmed that each PTX@HSA-CHP NPs and PTX@HSA NPs maintained spherical shapes (Fig. 4C).
Lengthy-term monitoring of the hydrodynamic diameter demonstrated that the NPs retained a constant particle measurement over 4 days. Nevertheless, past this era, the PDI elevated and exceeded 0.2, probably as a consequence of nanoparticle agglomeration, which is a typical situation with albumin-based nanoparticles. Notably, the incorporation of CHP didn’t trigger important adjustments in DLS measurements in comparison with PTX@HSA NPs, indicating that CHP conjugation didn’t adversely have an effect on the steadiness of the NPs (Fig. 4D). Moreover, in vitro drug launch research confirmed comparable cumulative launch profiles for each PTX@HSA-CHP NPs and PTX@HSA NPs, suggesting that the addition of CHP didn’t considerably alter the drug launch kinetics of the NPs (Fig. 4E).
Characterizations of therapeutic albumin NPs. A) Particle Measurement distribution and B) Zeta potential of PTX@HSA-CHP NPs and PTX@HSA NPs. C) Morphology of NPs noticed by TEM. The size bars have been 100 nm. D) Bodily Stability of the NPs have been measured by DLS. E) In vitro launch of PTX from PTX@HSA-CHP NPs and PTX@HSA NPs
Biosafety analysis of therapeutic NPs
To judge the biodistribution and potential toxicity of PTX@HSA-CHP NPs, we carried out HPLC evaluation to quantify PTX focus within the main organs of ApoE-/- mice at 1, 6, 12, and 24 h following intravenous injection of the PTX@HSA-CHP NPs. The outcomes confirmed that PTX initially accrued predominantly within the liver and kidney, reaching peak ranges at 6 h post-injection. Over time, the PTX focus ranges decreased as a consequence of hepatic metabolism and subsequent excretion, with practically clearance noticed inside 24 h (Determine S2A). These findings prompt that PTX was primarily cleared through hepatic metabolism after launch from NPs.
As well as, we carried out hematological evaluation and H&E staining of the primary organs (coronary heart, liver, spleen, lung and kidney) from the wholesome ApoE-/- mice at 1 day, 7 days and 14 days after the intravenous injection of the PTX@HSA-CHP NPs. As listed in Determine S2B, no important adjustments of the hemocyte check and features of liver and kidneys have been noticed after injection. Moreover, H&E staining demonstrated that the tissues retained regular buildings, with no observable organ injury or inflammatory lesions after therapy (Determine S2C). Collectively, these outcomes indicated that PTX@HSA-CHP NPs exhibited a good biosafety profile in ApoE-/- mice, supporting their potential for additional in vivo experiments.
Analysis of focused supply functionality and therapeutic impact in vivo
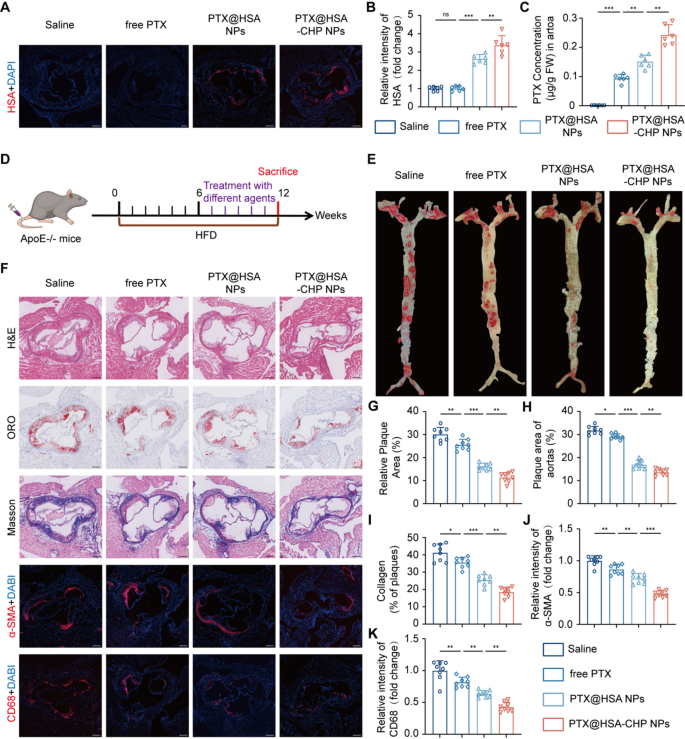
To judge the focused supply functionality of the NPs, atherosclerotic mice, which had been fed with a high-fat weight-reduction plan (HFD) for six weeks, have been handled with saline, free PTX, PTX@HSA NPs, and PTX@HSA-CHP NPs, respectively. Immunofluorescence staining outcomes revealed that the PTX@HSA-CHP NPs group exhibited the strongest fluorescence indicators inside atherosclerotic plaques within the murine aorta (PTX@HSA-CHP NPs vs. PTX@HSA NPs, 3.38 ± 0.52 vs. 2.62 ± 0.24, n = 6, P < 0.01; PTX@HSA NPs vs. free PTX, 2.62 ± 0.24 vs. 1.00 ± 0.13, n = 6, P < 0.001; free PTX vs. Saline, 1.00 ± 0.13 vs. 1.00 ± 0.12, n = 6, P > 0.05) (Fig. 5A and B). Constantly, HPLC evaluation confirmed that the focus of paclitaxel within the aortas of the PTX@HSA-CHP NPs group was considerably larger than different teams, regardless of administering the identical dose of paclitaxel (PTX@HSA-CHP NPs vs. PTX@HSA NPs, 0.2425 ± 0.0351 vs. 0.1512 ± 0.0219, n = 6, P < 0.01; PTX@HSA NPs vs. free PTX, 0.1512 ± 0.2189 vs. 0.0937 ± 0.0138, n = 6, P < 0.01; free PTX vs. Saline, 0.0937 ± 0.0138 vs. 0.0015 ± 0.0003, n = 6, P < 0.001) (Fig. 5C). These findings demonstrated that the conjugation with CHP markedly enhanced the plaque focusing on drug supply functionality of albumin NPs, making them a promising technique for focused therapy of atherosclerosis.
To additional consider the therapeutic efficacy of the NPs, AS mice fed with a HFD for six weeks have been randomly divided into 4 teams: saline, free PTX, PTX@HSA NPs, and PTX@HSA-CHP NPs. Every group acquired weekly intravenous injections of the respective therapies for six weeks (Fig. 5D). In comparison with the opposite group, AS mice handled with PTX@HSA-CHP NPs exhibited considerably diminished aortic plaque areas (PTX@HSA-CHP NPs vs. PTX@HSA NPs, 11.36 ± 2.15 vs. 16.09 ± 1.44, n = 8, P < 0.01; PTX@HSA NPs vs. free PTX, 16.09 ± 1.44 vs. 25.58 ± 2.35, n = 8, P < 0.001; free PTX vs. Saline, 25.58 ± 2.35 vs. 30.13 ± 2.98, n = 8, P < 0.01), indicating the notably therapeutic impact (Fig. 5E and G).
On the identical time, comparability of ORO-stained frozen sections of the aortic root supported these findings with an analogous pattern (Fig. 5F). It may very well be seen that the PTX@HSA-CHP NPs therapy group exhibited the bottom share of plaque space (PTX@HSA-CHP NPs vs. PTX@HSA NPs, 13.65 ± 1.38 vs. 16.96 ± 1.87, n = 8, P < 0.01; PTX@HSA NPs vs. free PTX, 16.96 ± 1.87 vs. 29.13 ± 1.17, n = 8, P < 0.001; free PTX vs. Saline, 29.13 ± 1.17 vs. 31.80 ± 1.75, n = 8, P < 0.05) (Fig. 5H). Moreover, collagen content material throughout the plaques, recognized by Masson staining, confirmed that mice injected with PTX@HSA-CHP NPs had the least collagen deposition in comparison with the opposite teams (PTX@HSA-CHP NPs vs. PTX@HSA NPs, 18.52 ± 2.71 vs. 25.53 ± 3.00, n = 8, P < 0.01; PTX@HSA NPs vs. free PTX, 25.53 ± 3.00 vs. 35.56 ± 3.01, n = 8, P < 0.001; free PTX vs. Saline, 35.56 ± 3.01 vs. 41.28 ± 4.98, n = 8, P < 0.05) (Fig. 5F and I). In the meantime, owing to the antiproliferative and anti inflammatory operate of paclitaxel, the relative fluorescence depth of α-SMA and CD68 have been considerably decreased in each the PTX@HSA NPs and PTX@HSA-CHP NPs teams in comparison with the saline and free PTX group (α-SMA : PTX@HSA-CHP NPs vs. PTX@HSA NPs, 0.48 ± 0.05 vs. 0.72 ± 0.08, n = 8, P < 0.001; PTX@HSA NPs vs. free PTX, 0.72 ± 0.08 vs. 0.86 ± 0.08, n = 8, P < 0.01; free PTX vs. Saline, 0.86 ± 0.08 vs. 1.00 ± 0.08, n = 8, P < 0.01; CD68: PTX@HSA-CHP NPs vs. PTX@HSA NPs, 0.43 ± 0.08 vs. 0.64 ± 0.05, n = 8, P < 0.01; PTX@HSA NPs vs. free PTX, 0.64 ± 0.05 vs. 0.82 ± 0.08, n = 8, P < 0.01; free PTX vs. Saline, 0.82 ± 0.08 vs. 1.00 ± 0.15, n = 8, P < 0.01) (Fig. 5F). Notably, PTX@HSA-CHP NPs exhibited superior anti-smooth muscle cell proliferation skill and the bottom diploma of macrophage infiltration, in line with the therapy results noticed within the ORO and H&E staining (Fig. 5J and Okay). Taken collectively, these outcomes highlighted that the conjugation of CHP considerably enhanced the therapeutic impact of the PTX@HSA NPs by bettering the focused drug supply skill, underscoring its potential for improved therapy of atherosclerosis.
Analysis of focused drug supply and therapeutic efficacy in vivo. A) Consultant immunofluorescence photos of atherosclerotic plaques, assessing albumin accumulation in plaques. B) Quantitative evaluation of relative depth of HSA in atherosclerotic plaques. C) Measurement of paclitaxel content material inside plaques. D) Schematic diagram of the therapeutic schedule. E) ORO staining of remoted aorta. F) Histological staining together with H&E, ORO and Masson staining, and immunofluorescence staining together with α-SMA and CD68 of aortic root. Quantitative evaluation of relative plaque space of the G) aorta and H) aortic root, and I) collagen of plaques. Quantitative evaluation of the fluorescence depth of J) α-SMA and Okay) CD68 in aortic roots. Experimental information have been expressed as imply ± SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significance. The size bars have been 200 μm