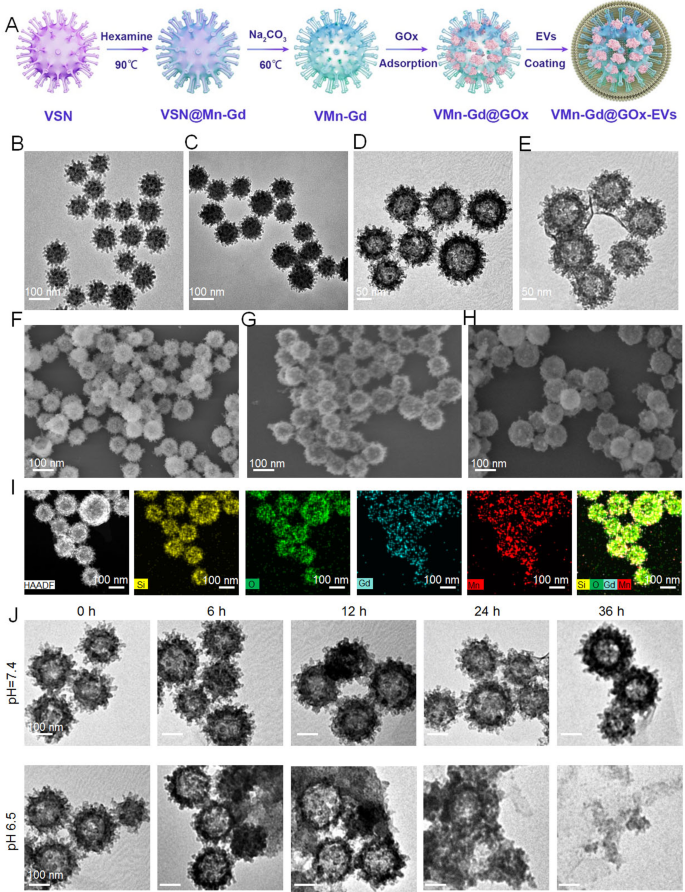
Preparation and characterization of the VMn-Gd@GOx-EVs
Right here, virus-mimic hole mesoporous Mn-Gd hybrid nanocarriers (VMn-Gd) have been synthesized using a standard exhausting template technique (Fig. 1A). Initially, the exhausting template, virus-mimic mesoporous SiO2 nanotemplate (VSN), was produced by way of a mono-micelle epitaxial progress methodology in a biphasic response system (water/cyclohexane) as beforehand reported. On this course of, CTAB and TEA served as surfactant and reductant, respectively, and have been dissolved in deionized water. TEOS, the silica precursor, was added to cyclohexane in a 1:4 quantity ratio and thoroughly dripped onto the aqueous section. After 72 h, VSN was obtained, exhibiting distinctive morphology and wonderful mono-dispersity with a really uniform measurement distribution (~ 100 nm). TEM and SEM pictures revealed that quite a few spinous nanotubes (roughly 5–10 nm) have been radially assembled on the spherical floor of VSN (Fig. 1B, F). Subsequently, Mn-Gd precursors, Mn(NO3)2•6H2O and Gd(NO3)3•6H2O in a 3:2 molar ratio, have been used to type a MnO2-Gd2O3 hybrid shell round VSN (VSN@Mn-Gd) underneath hydrothermal circumstances with weak alkali methylamine (Fig. 1C). The spinous nanotubes facilitated in situ nucleation of the Mn-Gd precursor hydrolysis. VSN was then eliminated utilizing 0.1 M NaOH, leading to distinctive hole Mn-Gd bimetallic oxide nanocarriers (VMn-Gd) with a uniform diameter of roughly 110 nm (Fig. 1D). Determine 1G reveals that VMn-Gd retains the virus-mimic morphology of VSN, together with the nanospikes on the spherical floor. Subsequently, GOx have been encapsulated in VMn-Gd and the loading quantity of GOx was decided utilizing a Bicinchoninic Acid (BCA) Protein Assay equipment. In VMn-Gd@GOx, the enzyme loading was discovered to be ∼0.21 ± 0.13 mg-GOx/mg-VMn-Gd with the loading effectivity as 17.35%. Lastly, VMn-Gd@GOx was coated with EV membranes (VMn-Gd@GOx-EVs) utilizing mild sonication at 4 °C adopted by filter extrusion. TEM confirmed the presence of the outer membrane layer, with the dimensions barely growing by 10 nm (Fig. 1E). SEM pictures indicated that the virus-like nanostructure of VMn-Gd@GOx grew to become blurred in VMn-Gd@GOx-EVs (Fig. 1H). These findings confirmed the profitable encapsulation EVs on the virus-like nanoagents. Excessive-angle annular darkish subject scanning electron microscopy (HADDF-SEM) was used to research elemental distribution, revealing homogeneously dispersed Si, O, Mn, and Gd components within the nanocarriers (Fig. 1I), with additional affirmation from elemental mapping evaluation in Fig. S1. Inspired by these outcomes, we evaluated the tumor microenvironment-responsive functionality of our nanoplatform. TEM pictures recorded after incubating Mn-Gd@GOx in PBS (1X) at pH 7.4 or 6.5 for various instances (Fig. 1J) confirmed no vital morphological adjustments at pH 7.4 even after 36 h, indicating stability underneath impartial circumstances. Nevertheless, VMn-Gd@GOx displayed a transparent time-dependent degradation at pH 6.5, with half of the nanoshells decomposing at 12 h and full collapse after 36 h. Notably, in comparison with conventional Gd-based nanosensitizers used for radiotherapy [44, 45], our VMn-Gd@GOx-EVs degrade underneath mildly acidic tumor microenvironment circumstances, releasing small particles round 10 nm after 36 h (Fig. 1J), which additional promotes tumor penetration. Excitingly, after incubation for 48 h at pH 6.5, the discharge effectivity of GOx was greater than 7 instances greater than that at pH 7.4 (Fig. S2). These outcomes reveal the nanoplatform’s sensitivity to acidic tumor microenvironments, making it appropriate for exact cargo supply, early analysis and the next synergistic remedy for tumors.
(A) A schematic illustration of the fabrication course of for VMn-Gd@GOx-EVs. TEM pictures showcasing (B) VSN, (C) VSN@Mn-Gd, (D) VMn-Gd, and (E) VMn-Gd@GOx-EVs. SEM pictures displaying (F) VSN, (G) VMn-Gd, and (H) VMn-Gd@GOx-EVs. (I) HADDF pictures illustrating the uniform elemental distribution of Si, O, Mn, and Gd in VMn-Gd@GOx. (J) TEM pictures of VMn-Gd@GOx after being immersed in buffers with completely different pH ranges (6.5, 7.4) for numerous durations
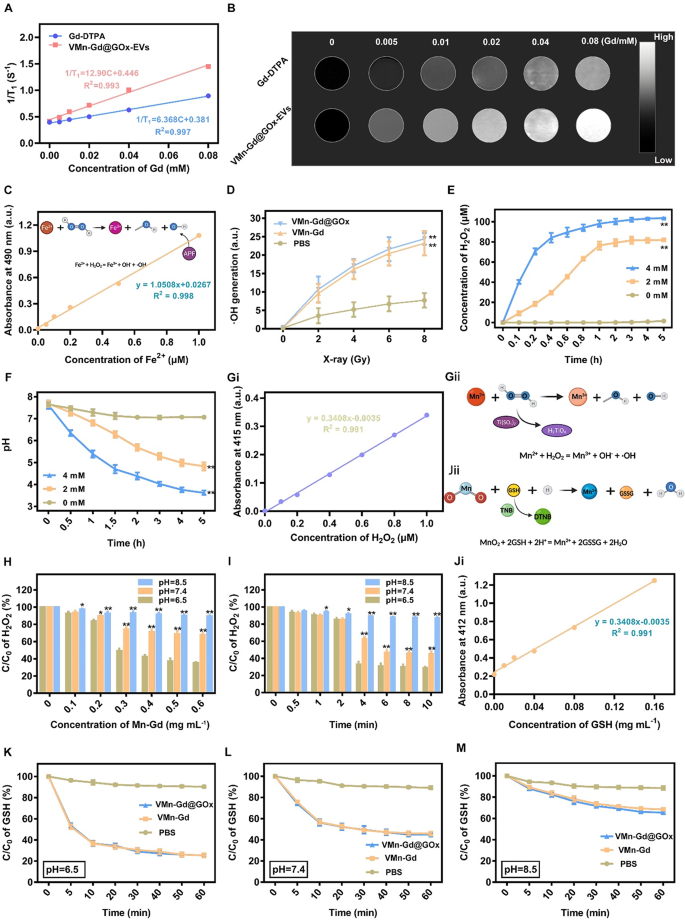
MRI functionality, GSH consumption, GOx depletion and ROS era in bulk answer
Owing to Mn and Gd ions might be triggered to launch in tumor acid microenvironment, then we assessed the efficiency of T1-weighted contrast-enhanced MRI, VMn-Gd@GOx-EVs and gadopentetic acid (Gd-DTPA) with completely different Gd3+ concentrations have been imaged utilizing a 9.4 T MRI system. As proven in Fig. 2A, Mn-Gd@GOx-EVs confirmed extra vital T1 MRI alerts than Gd-DTPA, which was the commonest MRI distinction agent on the clinic. The r1 relaxivity of Mn-Gd@GOx-EVs was 12.90 mM−1 s−1, which was ~ 2-fold greater than that of Gd-DTPA (6.368 mM−1 s−1) (Fig. 2B). Moreover, the brightness of T1-weighted MRI pictures elevated with Gd3+ focus, suggesting that Gd3+ in our naonsystem might successfully improve the MRI alerts for correct tumor delineating. Subsequently, to guage the radio-enhancement impact of VMn-Gd@GOx, an aminophenyl fluorescein (APF) probe was used to seize •OH (Fig. 2C). As proven in Fig. 2D, VMn-Gd and VMn-Gd@GOx generated equal portions of •OH that prominently greater than PBS upon X-ray irradiation (8 Gy) with ~ 2-fold relative enhancements. As well as, the manufacturing of •OH was associated to the radiation dose, demonstrating that X-rays have been the important thing issue for inducing •OH era. Subsequently, the enzyme exercise of VMn-Gd@GOx (Fig. S3) was firstly examined underneath numerous glucose circumstances. Outcomes confirmed {that a} excessive glucose degree induced extra H2O2 era inside 5 h incubation reaching the plateau. Notably, the generated H2O2 rapidly reached an equilibrium inside solely 0.6 h, demonstrating that the VMn-Gd@GOx possessed robust and fast glucose decomposition functionality for H2O2 era (Fig. 2E). The generate pH variation throughout glucose consumption additionally confirmed the same tendency, the place a excessive glucose degree led to a higher pH lower (from 7.4 to three.5) (Fig. 2F). The pH lower brought on by the manufacturing of gluconic acid might set off the degradation of VMn-Gd@GOx, which was demonstrated by the TEM pictures (Fig. 1J). Moreover, this induced decrease pH situation (4 ~ 5) might be facilitate for the next Fenton-like response of Mn ions. Collectively, both pH decline or the H2O2 enhance can considerably mediating the biodegradation of Mn-Gd@GOx and the next •OH era, making the as-prepared VMn-Gd@GOx a wonderful nanocarrier for tumor acidity-responsive drug supply and oxidative remedy. Therefore, the produced •OH via a Fenton-like response was latterly appraised in numerous of pH values. To guage the consumption of H2O2 by VMn-Gd, titanium sulphate (H2TiO4) was used as an indicator since H2TiO4 might be degraded by H2O2 accompanied by the manufacturing of yellow merchandise (Ti(SO4)2) (Fig. 2Gi, Gii, S4). Apparently, information confirmed that the exhaustion of H2O2 had the focus of VMn-Gd dependent method, particularly when pH worth was set at weak acid situation with 2.5 instances greater H2O2 decomposition effectivity than that of pH 8.5 group (Fig. 2H). The focus of H2O2 reached equilibrium at these three pH circumstances when the focus of VMn-Gd was set as 0.5 mg mL−1. As well as, VMn-Gd and H2O2 can rapidly attain saturation inside ~ 4 h at pH 6.5, whereas, the response price considerably decreased and the saturated time was ~ 6 h at pH 7.4 group. Nevertheless, at pH 8.5, there may be indiscernible H2O2 decomposition (Fig. 2I). Furthermore, MnO2 can even react with intracellular GSH to provide Mn2+. Therefore, to guage exhaustive skill of VMn-Gd@GOx towards GSH underneath completely different pH values, right here, 5,5’-Dithiobis-(2-nitrobenzoic acid) (DTNB) was used as an indicator since DTNB might be degraded by GSH accompanied by the manufacturing of yellow merchandise (TNB) (Fig. 2Ji, Jii, S5). Clearly, we discovered that VMn-Gd@GOx and VMn-Gd exhibit the identical tendency to eat GSH that overwhelmingly greater than PBS handled group (Fig. S6). Additional, outcomes additionally confirmed that, in distinction with pH 7.4 and pH 8.5 teams, GSH might be considerably decomposed by VMn-Gd@GOx at pH 6.5, particularly inside 10 min of incubation (Fig. 2Ok-M, S7). In short, all above findings demonstrated that our Mn-Gd based mostly nanoplatform might be utilized for MRI distinction agent and it is ready to induce ROS storm underneath X-ray, excessive glucose in addition to excessive GSH circumstances. This programmed nanoagents held nice potential for the next tumor tissues analysis and suppression by way of synergistic RT and oxidative remedy.
The r1 relativities (A) and in vitro MRI pictures (B) of Gd-DTPA and VMn-Gd@GOx underneath completely different concentrations. (C) Commonplace curve of AFP vs. numerous concentrations of Fe2+ for evaluating •OH era underneath X-ray irradiation. (D) Assessments of •OH era in VMn-Gd@GOx, VMn-Gd and PBS underneath numerous energy density of X-ray. (E) The generated H2O2 concentrations and (F) fluctuation of pH values after incubated VMn-Gd@GOx with completely different dose of glucose for numerous durations. (Gi) Commonplace curve of Ti(SO4)2 vs. numerous concentrations of H2O2 for evaluating •OH era underneath Mn ions catalysis. Schematic illustration of (Gii) H2TiO4 for •OH detection and (Jii) DNTB for GSH consumption. Residual H2O2 percentages after incubated with VMn-Gd@GOx (H) at completely different dose or (I) for numerous intervals underneath pH 6.5, pH 7.4 and pH 6.5 circumstances. (Ji) Commonplace curve of TNB vs. numerous concentrations of GSH. The residual GSH content material after treating VMn-Gd@GOx, VMn-Gd and PBS with (Ok) pH = 6.5, (L) pH = 7.4 and (M) pH = 8.5 buffers for various minutes. The information are introduced because the imply ± SD (n = 3), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01
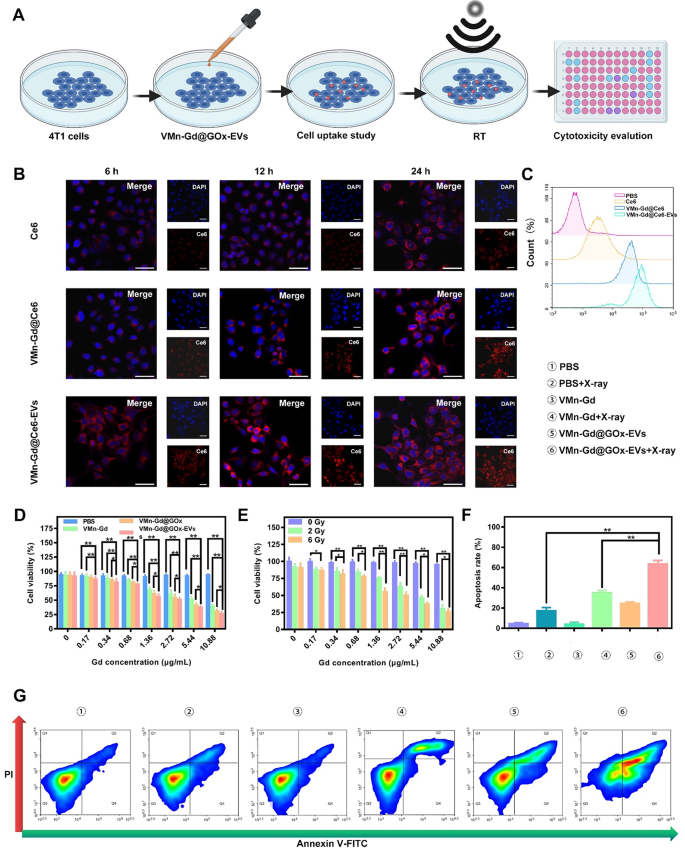
In vitro tumor cell killing efficacies of Mn-Gd@GOx-EVs
We have been motivated to research the therapeutic mechanism and efficacy of VMn-Gd@GOx-EVs on the mobile degree (Fig. 3A). Earlier than assessing the cytotoxic results induced by X-rays, we evaluated the intracellular uptake of those therapeutic nanomaterials utilizing the 4T1 breast carcinoma cell line. On this case, GOx was changed by the fluorophore Ce6, ensuing within the formation of VMn-Gd@Ce6-EVs. This allowed the visualization of the internalization course of by way of CLSM and move cytometry. As proven in Fig. 3B, C and S8, the intracellular pink fluorescence of VMn-Gd@Ce6-EVs was distinctively greater than that of free Ce6 and VMn-Gd@Ce6 (Ce6-labeled VMn-Gd) in any respect examined time factors (6, 12, and 24 h). Moreover, the endocytosis effectivity of VMn-Gd@Ce6 was considerably greater in comparison with free Ce6. This enchancment might be attributed to the virus-inspired tough floor of VMn-Gd@Ce6, which strongly interacts with cell membranes, facilitating the entry of Ce6 into the cells. Moreover, the EVs coating enhanced mobile endocytosis, highlighting the potential of EVs as efficient automobiles for intercellular supply. The superior compatibility of the EVs with the cell membrane may be the important thing issue driving this enhanced internalization. These findings additional confirmed that VMn-Gd@GOx-EVs is an optimum formulation for environment friendly nanotherapeutic supply and reveals nice promise as a biomimetic drug supply system. After establishing the endocytic properties of the nanomaterials, we evaluated the cytotoxicity of VMn-Gd@GOx-EVs underneath X-ray irradiation utilizing a cell counting kit-8 (CCK-8) assay. As seen in Fig. 3D, PBS-treated tumor cells exhibited undiscoverable toxicity upon X-ray publicity, demonstrating the biosafety of X-rays on the examined energy density (6 Gy). In distinction, cells uncovered to VMn-Gd-based formulations exhibited elevated cytotoxicity in a dose-dependent method. Notably, the VMn-Gd@GOx-EVs group confirmed the best cell killing skill in comparison with the VMn-Gd and VMn-Gd@GOx teams when all teams obtained X-ray illumination. This enhanced cytotoxicity could possibly be attributed to the robust endocytic exercise of the EVs. Moreover, the cytotoxic impact was additional amplified by growing the X-ray energy density (Fig. 3E). At 6 Gy, 73.88% of cells within the VMn-Gd@GOx-EVs group (10.88 µg•mL−1 Gd equal) have been killed. This X-ray energy density was utilized in all subsequent in vitro and in vivo research for tumor cell elimination. Furthermore, apoptosis evaluation was carried out utilizing the Annexin V-FITC/PI co-staining assay (Fig. 3G). The outcomes confirmed no detectable apoptosis in PBS, VMn-Gd, or Mn-Gd@GOx-EVs-treated tumor cells, confirming the biocompatibility of those formulations in vitro. VMn-Gd + X-ray remedy additionally exhibited a average cell killing impact (38.29%), indicating notable radiotherapy effectivity. Importantly, VMn-Gd@GOx-EVs handled with X-ray irradiation confirmed the best apoptosis price (61.20%), which aligned with the earlier CCK-8 outcomes (Fig. 3F). In abstract, VMn-Gd oxidative nanoagents might exhausted GSH for manganese ions (Mn2+) and O2 era, thereby enhancing RT effectivity for •OH manufacturing. GOx can induce pH decline and H2O2 enhance that subsequent •OH generated underneath Mn2+ catalysis. Subsequently, ROS storm was produced in cytoplasm for efficient tumor cell killing by way of synergistic RT and oxidative remedy.
(A) Schematic illustration of the remedy protocol for VMn-Gd@GOx-EVs in 4T1 cells. (B) CLSM pictures exhibiting 4T1 cells after incubation with free Ce6, VMn-Gd@Ce6, or VMn-Gd@Ce6-EVs for six, 12, and 24 h, all at equal Gd concentrations. The quantity of Ce6 was matched throughout all teams, together with free Ce6, VMn-Gd@Ce6, and VMn-Gd@Ce6-EVs. Scale bar represents 30 μm. (C) Movement cytometry evaluation of mobile uptake in 4T1 cells after 24-hour publicity to free Ce6, VMn-Gd@Ce6, or VMn-Gd@Ce6-EVs underneath the identical Gd focus. (D) Cell viability evaluation of 4T1 cells post-treatment with PBS, VMn-Gd, VMn-Gd@GOx, or VMn-Gd@GOx-EVs underneath X-ray irradiation (6 Gy) throughout numerous Gd concentrations. (E) Analysis of the cytotoxicity of VMn-Gd@GOx-EVs at completely different Gd ranges with various doses of X-ray (0, 3, and 6 Gy). (F-G) Quantification of apoptosis in 4T1 cells following publicity to completely different remedy formulations utilizing Annexin V-FITC/PI twin staining. Outcomes are proven as imply ± SD (n = 3). Statistical significance was analyzed utilizing one-way ANOVA adopted by Bonferroni correction: *P < 0.05, **P < 0.01
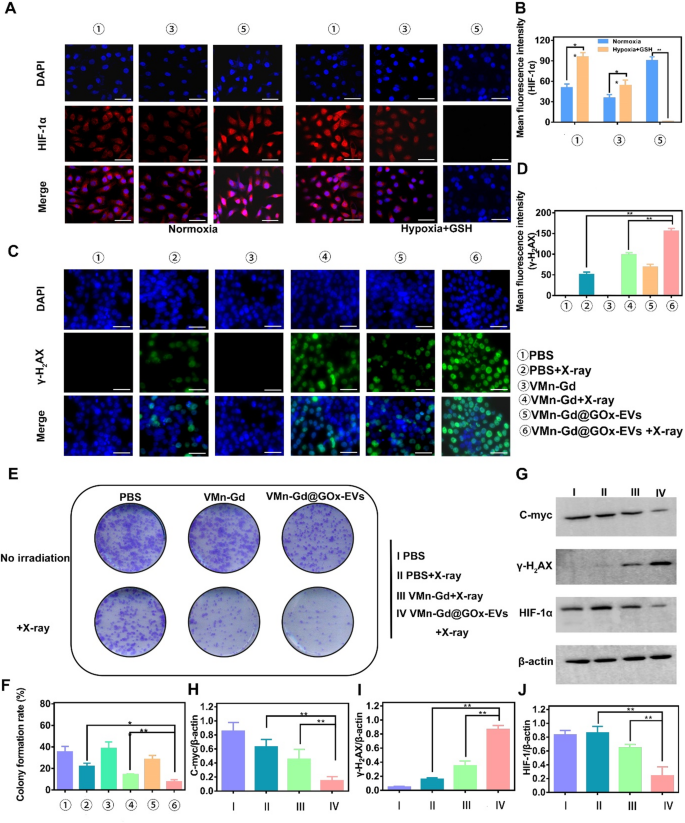
Undoubtedly, GOx particularly catalyzes the oxidation of β-D-glucose underneath excessive O2 situation. Subsequently, to evaluate the catalytic exercise of GOx, a hypoxia-inducible issue 1-alpha (HIF-1α) probe was employed to guage the extent of hypoxia on the mobile degree. As proven in Fig. 4A, in stark distinction to the minimal ranges of HIF-1α detected in normoxia and PBS-treated 4T1 cells, a strikingly intense pink fluorescence sign was noticed in cells subjected to incubation with VMn-Gd@GOx-EVs for 12 h with X-ray irradiation, profoundly demonstrating the strikingly excessive glucose consumption. This compelling commentary indicated a major up-regulation of intracellular HIF-1α expression throughout the hypoxic milieu. However, a considerably decreased expression of HIF-1α was then discerned in cells subjected to incubation with VMn-Gd@GOx-EVs plus X-ray shining for greater than 24 h (Fig. 4B). These findings might be attributed to huge Mn ions launch and excessive GSH ranges in tumor microenvironment, mediating O2 era. As well as, we meticulously assessed the expression ranges of HIF-1α, using Western blot evaluation for exact analysis. As illustrated in Fig. 4G, the bottom focus of HIF-1α throughout the cytoplasm was prominently noticed within the VMn-Gd@GOx-EVs group subjected to X-ray irradiation, aligning seamlessly with tendencies recognized in prior research. This commentary underscored a outstanding mitigation of hypoxia, attributable to the O2 era facilitated by VMn-Gd catalysis. Importantly, hypoxia reduction with HIF-1α down-regulation at all times has constructive correlation with radio-sensitization that induced the rise in ROS manufacturing ranges, accompanying by enhanced tumor cell toxicity by way of DNA damage-induced cell demise pathways. Following intently, the phosphorylated protein γ-H2AX is particularly localized at websites of DNA double-strand breaks and is esteemed as a pivotal biomarker for DNA injury induced by ionizing radiation. Consequently, to additional consider the radio-sensitization potential of VMn-Gd@GOx-EVs, the degrees of γ-H2AX inside mobile nuclei have been meticulously assessed using a DNA injury assay equipment, adopted by visualization via CLSM. As illustrated in Fig. 4C, the VMn-Gd@GOx-EVs mixed with X-ray remedy exhibited a markedly extra intense inexperienced fluorescence in comparison with different formulations. Notably, the quantitative evaluation of DNA injury ranges inside this group was considerably elevated when juxtaposed with each the PBS + X-ray and the standalone VMn-Gd@GOx-EVs teams. The density of γ-H2AX, as proven in Fig. 4D, underscores the numerous DNA injury induced by the VMn-Gd@GOx-EVs + X-ray group. Moreover, radio-cytotoxicity usually mediates DNA fragmentation and obstructs DNA replication, thereby essentially impeding clonal cell proliferation. Right here, the formation of colonies is illustrated in Fig. 4E, F. The VMn-Gd@GOx-EVs mixed with X-ray remedy demonstrated a outstanding skill to decrease the clonal formation price to roughly 7.73%. This represents a major discount, being 2.28-fold and 5.09-fold decrease than that noticed within the PBS + X-ray group (roughly 21.93%) and the only Mn-Gd@GOx-EVs group (round 38.33%), respectively. We have been prompted to evaluate the expression ranges of γ-H2AX, alongside the proliferation-associated protein c-myc, which have been meticulously evaluated via Western blot evaluation. As anticipated, the best focus of γ-H2AX and the bottom expression of c-myc throughout the cytoplasm have been distinctly noticed within the VMn-Gd@GOx-EVs subjected to X-ray irradiation (Fig. 4G, H-J, S9). These findings align seamlessly with tendencies established in prior analysis. Briefly, all information additional substantiated that VMn-Gd@GOx-EVs exhibited a outstanding radio-sensitization impact, considerably impeding the proliferation of cancerous cells. These positions themed as a promising candidate for nanoagents of radio-sensitization remedy.
(A) CLSM pictures and quantitative fluorescence intensities (B) of HIF-α in 4T1 cells handled with completely different formulations. Cells have been cultured underneath normoxic circumstances (left) and hypoxia with excessive GSH circumstances (proper). (C) CLSM pictures and (D) common fluorescence depth (γ-H2AX) of 4T1 cells handled with numerous formulations to evaluate DNA fragmentation and nuclear condensation utilizing γ-H2AX staining. (E) Consultant pictures and (F) colony formation price from a colony formation assay. (G) Impression of VMn-Gd@GOx-EVs mixed with X-ray irradiation (6 Gy) on the expression ranges of γ-H2AX, HIF-1α, and C-myc proteins in 4T1 cells. Corresponding γ-H2AX/β-actin, HIF-1α/β-actin, and C-myc/β-actin ratios in 4T1 cells following numerous remedies. Scale bar represents 100 μm. The information are introduced because the imply ± SD (n = 3), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01
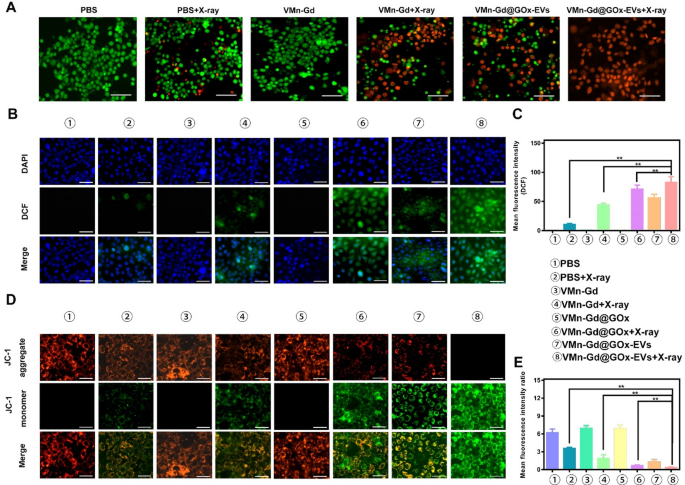
To additional assess tumor cell killing, a reside/lifeless cell staining assay was carried out utilizing VMn-Gd@GOx-EVs mixed with X-ray irradiation. In line with the apoptosis information, a lot of the tumor cells have been stained with pink fluorescence, indicating cell demise. PBS, VMn-Gd, and VMn-Gd@GOx-EVs teams confirmed minimal toxicity, with cells emitting inexperienced fluorescence, confirming minimal influence on regular cells (Fig. 5A). Within the VMn-Gd + X-ray group, nevertheless, vital numbers of lifeless cells have been noticed, according to the apoptosis outcomes, suggesting that the only RT method had some limitations in eradicating malignant cells. In distinction, VMn-Gd@GOx-EVs mixed with X-ray irradiation exhibited robust tumor-specific cytotoxic results, pushed by a mixture of ROS era from tumor cell focusing on, RT, a Fenton-like response and GSH depletion. Constructing on these findings, the era of intracellular ROS was additional examined utilizing DCFH-DA staining (Fig. 5B). CLSM pictures revealed that VMn-Gd@GOx-EVs with X-ray publicity produced a considerably greater inexperienced fluorescence, roughly 6 instances higher than the ROS ranges within the PBS group with X-ray irradiation (Fig. 5C). Prominently, ROS ranges have been greater within the VMn-Gd@GOx-EVs + X-ray group in comparison with the VMn-Gd@GOx + X-ray group. The exact tumor tissues focusing on of EVs, the discount of pH and the era of H2O2 by GOx that essentially promoted the Fenton-like response of Mn ions. Additional, each GSH consumption and hypoxia assuaging had enhanced the radiotherapy effectivity upon X-ray irradiation. Consequently, in comparison with different remedy methods, all above benefits contributed to the environment friendly cell killing by way of ROS storm in VMn-Gd@GOx-EVs + X-ray group. Mitochondria current a great goal for enhancing ROS-based assaults in most cancers remedy. Nanocarriers with a constructive cost are thought-about optimum for focusing on mitochondria. By leveraging the distinctive traits of mitochondria, the focused supply of Mn2+ to this organelle can successfully maximize the era of ROS for oxidative remedy. To visualise this, we used the JC-1 probe, which aggregates and emits pink fluorescence when the mitochondrial membrane potential is unbroken. Upon depolarization, it transitions to a monomeric type, emitting inexperienced fluorescence. As proven in Fig. 5D, E, the pink fluorescence progressively decreased whereas the inexperienced fluorescence elevated in cells handled with VMn-Gd@GOx-EVs adopted by X-ray irradiation. Importantly, the pink/inexperienced fluorescence depth ratio was notably decreased within the VMn-Gd@GOx-EVs + X-ray group in comparison with the opposite teams. These CLSM outcomes verify that the Mn-Gd bimetallic nanoplatform successfully induces ROS era underneath X-ray irradiation and Fenton-like catalysis, resulting in vital cytotoxic results on tumor cells.
(A) Stay-dead cell staining of 4T1 cells handled with numerous formulations and X-ray irradiation (6 Gy) utilizing calcein-AM/PI assay (scale bar represents 60 μm). (B) CLSM pictures and (C) corresponding quantitative fluorescence intensities of 4T1 cells handled with completely different formulations, adopted by X-ray irradiation (6 Gy), to evaluate ROS era by way of DCFH-DA staining (scale bar represents 30 μm). (D) CLSM pictures and (E) quantitative fluorescence intensities of 4T1 cells handled with numerous formulations underneath X-ray publicity (6 Gy) to guage mitochondrial membrane potential utilizing JC-1 staining (scale bar represents 30 μm). The information are introduced because the imply ± SD (n = 3), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01
In vivo breast most cancers focusing on
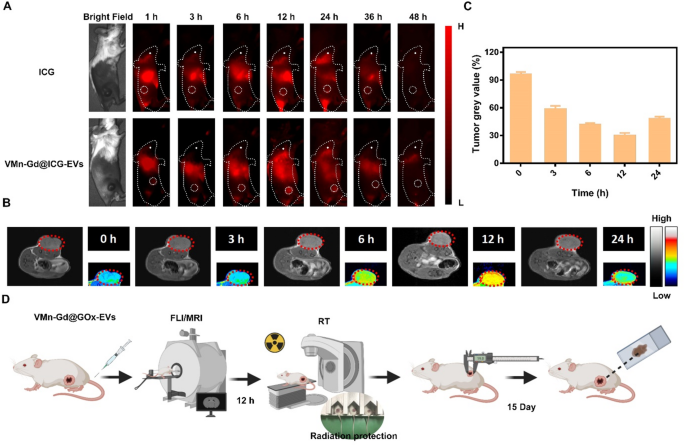
The encouraging radiotherapy and tumor focusing on capabilities noticed in vitro prompted an in vivo examine to evaluate the therapeutic potential of Mn-Gd@GOx-EVs. On this examine, 4T1 cells, a murine breast carcinoma mannequin recognized for fast malignant progress and in depth multi-organ metastasis in BALB/c mice, have been used. Subcutaneous 4T1 tumor-bearing mice have been chosen for this experiment. To observe the in vivo biodistribution and tumor focusing on skill of VMn-Gd@ICG-EVs, we changed GOx with Indocyanine inexperienced (ICG), a near-infrared (NIR) dye, within the virus-like nanoparticles (VMn-Gd@ICG-EVs). Fluorescence imaging was carried out utilizing a 50 ms publicity time, 808 nm laser excitation, and a 1000 nm long-pass filter. As proven in Fig. 6A, the preliminary tumor location was clearly distinguishable from surrounding muscle tissue at 6 h post-administration of VMn-Gd@ICG-EVs. The fluorescence depth considerably elevated, reaching its peak at 12 h after tail-vein injection. At 24 h, the NIR sign within the tumor started to fade, with full attenuation noticed at 36 h (Fig. 6A). In distinction, the ICG-only group confirmed a lot weaker fluorescence on the tumor web site all through the imaging interval with three-fold decrease NIR depth than VMn-Gd@ICG-EVs handled group at 24 h (Fig. S10). On this examine, Mn/Gd ions usually are triggered to launch underneath weak acid tumor microenvironment. These launched ions can successfully work together with surrounding water molecules in tumor tissues, altering the native magnetic resonance properties, thereby enhancing the MRI sign. In gentle of this, the in vivo tumor focusing on skill was assessed over a 24-hour interval utilizing MRI after the administration of VMn-Gd@GOx-EVs by way of the tail vein. Sturdy T1-weighted MR alerts have been noticed on the tumor web site, beginning at 3 h post-injection, and reached peak depth at 12 h (Fig. 6B, C), according to the NIR fluorescence imaging outcomes (Fig. 6A). Markedly, even at 24 h, a major MR sign remained across the tumor space, confirming that VMn-Gd@GOx-EVs successfully gathered within the tumor microenvironment (Fig. 6B, C). This discovering means that the Mn-Gd-based nanoplatform can function an MRI distinction agent for potential scientific purposes. Primarily based on these observations, X-ray irradiation for RT ought to be carried out 12 h after nanoplatform injection to attain optimum tumor suppression (Fig. 6D).
(A) In vivo NIR fluorescence imaging of 4T1 tumor-bearing mice at numerous time factors following the injection of both VMn-Gd@ICG-EVs or ICG. (B) In vivo MRI scans and tumor grayscale values of 4T1 tumor-bearing mice at numerous time factors after injection of VMn-Gd@GOx-EVs. (C) Tumor gray worth at completely different time intervals after remedy with VMn-Gd@ICG-EVs. (D) A flowchart for in vivo tumor imaging adopted by tumor remedies. The information are introduced because the imply ± SD (n = 4), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01
In vivo radiosensitization remedy of antitumor impact of VMn-Gd@GOx-EVs with X-ray irradiation
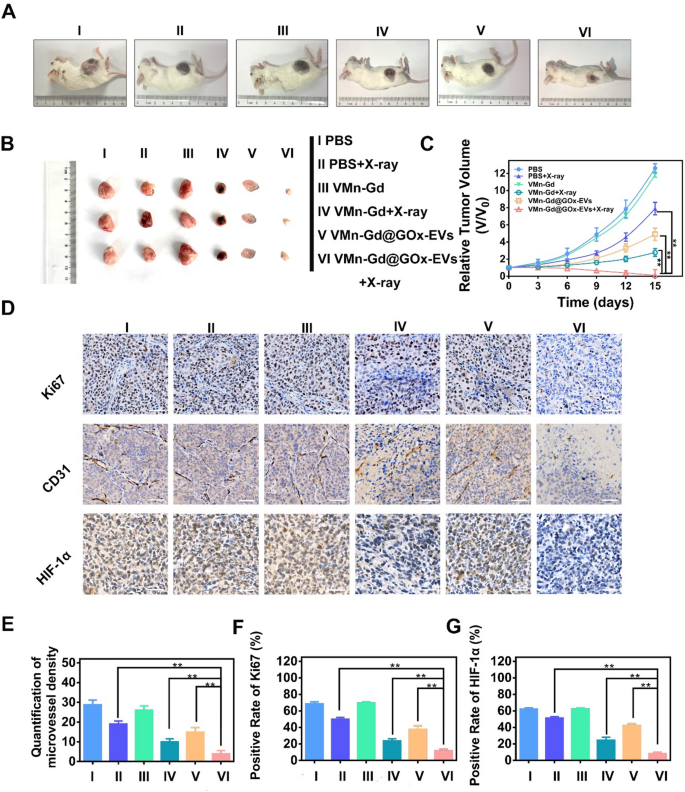
Lastly, the therapeutic efficacy of radiosensitization remedy using VMn-Gd@GOx-EVs along with X-ray irradiation was meticulously assessed via the institution of a subcutaneous transplantation mannequin in BALB/c mice. A collection of three tail-vein injections comprising numerous formulations have been administered on days 1, 3, and 5. Following this routine, X-ray publicity (6 Gy) was executed on the tumors inside three of those teams at 12 h post-injection (Fig. S11). The tumor progress volumes have been monitored each three days, clearly, the parallelism of tumor inhibition tendencies was tracked. As illustrated in Fig. 7A-C and S12, the tumors throughout the PBS and VMn-Gd cohorts exhibited a fast proliferation. In stark distinction, all VMn-Gd-based teams subjected to X-ray irradiation demonstrated a notable diploma of tumor progress inhibition, indicating that the ROS manufacturing induced by X-ray irradiation might considerably impede the proliferation of tumor lots. Strikingly, compared to the PBS + X-ray and VMn-Gd + X-ray cohorts, the VMn-Gd@GOx-EVs + X-ray group demonstrated a markedly enhanced inhibitory impact on tumor proliferation, exhibiting the smallest tumor sizes. This outstanding consequence might be attributed to the radiosensitization properties of VMn-Gd mixed with the EVs’ camouflage and the O2 era that facilitated prominently enhanced the RT effectivity. Moreover, it’s significantly noteworthy that GOx and GSH depletion considerably amplified ROS catalyzed efficacy whereas concurrently impeding some extent of tumor progress in VMn-Gd@GOx-EVs handled mice. The staining of Ki67, CD31, and HIF-1α is esteemed as pivotal assays for evaluating the efficacy of radiation remedy. On this context, we carried out immunohistochemical staining on sections of tumor tissue. The outcomes have been strikingly evident within the Ki67-stained slides (Fig. 7D). Particularly, when juxtaposed with various formulations, a outstanding discount starting from two to fourfold in Ki67 ranges was noticed inside malignant breast tumors following the remedy with VMn-Gd@GOx-EVs mixed with X-ray publicity (Fig. 7E). Moreover, radiation possesses the outstanding skill to orchestrate the obliteration of blood vessels inside tumors, thereby impeding tumor proliferation. The presence of intratumoral blood vessels is often inferred from the expression of CD31 on the floor of endothelial cells. As illustrated in Fig. 7D, F, the proportion of CD31-positive cells throughout the tumors of the VMn-Gd@GOx-EVs + X-ray cohort was markedly diminished in comparison with these noticed in different experimental teams. These findings compellingly reveal that the mixture of VMn-Gd@GOx-EVs and X-ray irradiation can successfully obliterate intratumoral blood vessels, thereby obstructing the vascular provide throughout the tumor and impeding its continued proliferation. As well as, the VMn-Gd@GOx-EVs + X-ray demonstrated a outstanding capability to alleviate hypoxia, thereby considerably augmenting the efficacy of radiotherapy. We additional investigated the expression ranges of HIF-1α. As illustrated in Fig. 7D, G, the proportion of intra-tumoral HIF-1α-positive cells throughout the VMn-Gd@GOx-EVs + X-ray group was conspicuously decrease than that noticed within the different experimental teams. These findings suggest that the radiosensitizing impact facilitated by VMn-Gd@GOx-EVs might considerably improve the tumor ablation outcomes of unparalleled ROS storm by way of RT and Fenton-like catalysis. In the meantime, the elevated ROS ranges within the cytoplasm are vital for inducing immunogenic cell demise, the place apoptotic tumor cells launch tumor-associated antigens that activate dendritic cells (DC) maturation, finally serving to to stop tumor recurrence and metastasis [46,47,48,49]. To guage this course of, the expression of CD80 and CD86, markers of DC maturation within the tumor, was analyzed utilizing move cytometry. Remarkably, the outcomes demonstrated that the best ranges of CD80+/CD86+ DC have been noticed following remedy with VMn-Gd@GOx-EVs + X-ray, surpassing all different remedy teams (Fig. S13), indicating that this method successfully promotes DC maturation that may be additional utilized for immunotherapy.
(A) Consultant pictures of 4T1 tumor-bearing mice on the fifteenth day after completely different remedies. (B) Consultant pictures of stable tumors after completely different remedies. (C) Tumor quantity variations after completely different remedies for 15 days. (D) Immunohistochemical staining of Ki67, CD31 and HIF-1α ranges in several handled tumor tissues at day 7. (E) Quantification of microvessel density, (F) Optimistic price of Ki67, and (G) constructive price of HIF-1α after completely different administrations at day 7. The information are introduced because the imply ± SD (n = 4), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01
Biosafety analysis
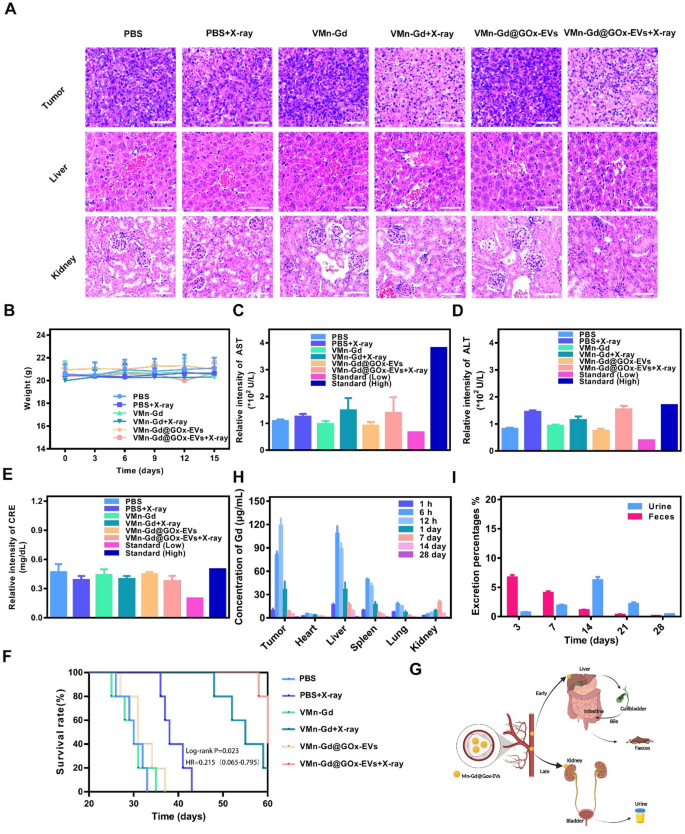
Biosafety represents a paramount concern within the preclinical software of nanotherapeutics. Consequently, complete assessments have been undertaken, encompassing physique weight measurements, histological examination of main organs by way of H&E staining, in addition to routine serum analyses and biochemical evaluations. As proven in Fig. 8A, considerably tumor cell injury was present in VMn-Gd@GOx-EVs + X-ray handled tumor, exhilaratingly, negligible tissue injury and fragmented cell membranes have been noticed within the principal organs, together with the guts, liver, spleen, lungs, and kidneys (Fig. S14). Moreover, not one of the mice exhibited any vital loss in physique weight all through the whole period of the remedy interval (Fig. 8B). To additional verify whether or not VMn-Gd@GOx-EVs + X-ray induced acute toxicity, blood samples have been meticulously collected from mice on the fifteenth day following remedy for complete biosecurity evaluation. As illustrated in Fig. 8C-E and S15, the principal serum enzyme ranges (together with AST, ALT, and CRE) in addition to blood cell counts encompassing WBC, NE, LY, RBC, HGB, and PLT have been constantly maintained inside commonplace physiological ranges. Collectively, these findings underscored the negligible systemic toxicity related to the VMn-Gd@GOx-EVs plus X-ray remedy group. Subsequently, we rigorously monitored the survival charges of mice subjected to varied therapeutic interventions. As proven in Fig. 8F, almost all BALB/c mice bearing mammary tumors within the PBS group succumbed by day 30 post-treatment. In stark distinction, the survival price of mice administered with VMn-Gd@GOx-EVs mixed with X-ray remedy was considerably prolonged past 60 days. This stands in marked comparability to the PBS + X-ray group, which exhibited a survival period of merely 40 days. This outstanding discovering might be attributed to the commendable ROS storm for tumor eradication. Moreover, to elucidate the in vivo biodistribution of VMn-Gd@GOx-EVs, we meticulously harvested the principal organs and tumors from mice following intravenous administration of nanoparticles at numerous time intervals. The concentrations of Gd3+ have been subsequently quantified utilizing inductively coupled plasma mass spectrometry (ICP-MS) (Fig. 8G, S16). In the course of the preliminary 6 h following intravenous injection, VMn-Gd@GOx-EVs predominantly localized throughout the liver and spleen (Fig. 8H, S17-S21). In distinction, a considerable amount of nanoagents exhibited vital accumulation on the tumor web site, peaking roughly on the 12-hour mark (Fig. S22). Subsequently, there was a notable decline in VMn-Gd@GOx-EVs throughout the examined organs as time progressed. In parallel, to elucidate the excretion dynamics of VMn-Gd@GOx-EVs in murine fashions, we quantified the degrees of Gd3+ current in feces and urine collected post-injection of nanoparticles at numerous intervals. Within the preliminary phases, VMn-Gd@GOx-EVs that gathered within the liver have been predominantly excreted by way of bile into feces. By the seventeenth day, the degraded nanoparticles could possibly be eradicated via the kidneys, leading to urine formation (Fig. 8I). On the fourteenth day, a peak was noticed in nanoparticle excretion via urine. Subsequently, this discharge progressively diminished and by the twenty eighth day, roughly 95% of VMn-Gd@GOx-EVs have been expelled at an elevated price via each feces and urine (Fig. S23, S24). These phenomena prompt that VMn-Gd@GOx-EVs additionally exhibited inherent biodegradability underneath physiological situation for very long time durations. EVs are capable of escape detection and clearance by phagocytic immune cells, whereas concurrently being effectively taken up by the supposed goal cells or tissues. Cytokine adjustments (together with IL-6, TNF-α and IL-1β) in blood have been examined after tail vein administration of VMn-Gd@GOx-EVs for various intervals. Exhilaratingly, in sharp distinction with regular group, indiscernible cytokine fluctuations have been found even after 48 h postinjection (Fig. S25), profoundly demonstration the unparalleled immune evasion skill after EVs camouflage. All outcomes demonstrated that VMn-Gd@GOx-EVs, when subjected to X-ray irradiation, might elicit a extremely favorable ROS era for tumor elimination, all whereas exhibiting negligible long-term uncomfortable side effects. This underscores their distinctive organic security and presents an optimum therapeutic outlook for oncological purposes. This examine additionally has the next limitations, akin to inadequate exploration of immune-mediated cell demise, lack of investigation into the blood half-life, and the absence of short-term acute toxicity of VMn-Gd@GOx-EVs at excessive focus. Furthermore, it has been reported that launched Gd3+ can bind with ATP in cytoplasm [50], and additional investigation is required to find out whether or not our nanoplatform induces an vitality deprivation impact inside cells.
(A) H&E stained pictures of tumor and very important organs after numerous remedies. (B) Physique weight fluctuated curves in the course of the tumor suppression interval. (C) ALT, (D) AST and (E) CRE ranges in several teams. (F) Survival charges of six completely different teams for 60 days. (G) Diagrammatic illustration of excretion share from urine and faeces after tail-vein injection of VMn-Gd@GOx-EVs for various intervals. (H) Bio-distribution of VMn-Gd@GOx-EVs after tail-vein injection for various intervals. (I) Excretion share of VMn-Gd@GOx-EVs after tail-vein injection for various intervals. The information are introduced because the imply ± SD (n = 4), with P-values decided by one-way ANOVA adopted by Bonferroni correction. *P < 0.05, **P < 0.01